Abstract
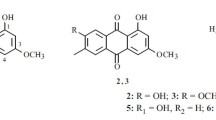
Through bioassay-guided fractionation, the EtOAc extract of a culture broth of the endophytic fungus Phoma species ZJWCF006 in Arisaema erubescens afforded a new α-tetralone derivative, (3S)-3,6,7-trihydroxy-α-tetralone (1), together with cercosporamide (2), β-sitosterol (3), and trichodermin (4). The structures of compounds were established on the basis of spectroscopic analyses. Compounds 1, 2, and 3 were obtained from Phoma species for the first time. Additionally, the compounds were subjected to bioactivity assays, including antimicrobial activity, against four plant pathogenic fungi (Fusarium oxysporium, Rhizoctonia solani, Colletotrichum gloeosporioides, and Magnaporthe oryzae) and two plant pathogenic bacteria (Xanthomonas campestris and Xanthomonas oryzae), as well as in vitro antitumor activities against HT-29, SMMC-772, MCF-7, HL-60, MGC80-3, and P388 cell lines. Compound 1 showed growth inhibition against F. oxysporium and R. solani with EC50 values of 413.22 and 48.5 μg/mL, respectively. Additionally, compound 1 showed no cytotoxicity, whereas compound 2 exhibited cytotoxic activity against the six tumor cell lines tested, with IC50 values of 9.3 ± 2.8, 27.87 ± 1.78, 48.79 ± 2.56, 37.57 ± 1.65, 27.83 ± 0.48, and 30.37 ± 0.28 μM, respectively. We conclude that endophytic Phoma are promising sources of natural bioactive and novel metabolites.





Similar content being viewed by others
References
Alvi KA, Nair B, Pu H, Ursino R, Gallo C, Mocek U (1997) Phomacins: three novel antitumor cytochalasan constituents produced by a Phoma sp. J Org Chem 62:2148–2151
Aveskamp MM, Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterize Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Bell AA, Stipanovic RD, Puhalla JE (1976) Pentaketide metabolites in Verticillium dahliae. Identification of (+) scytalone as a natural precursor to melanin. Tetrahedron 32:1353–1356
Corley DG, Miller-Wideman M, Durley RC (1994) Isolation and structure of harzianum A: a new trichothecene from Trichoderma harzianum. J Nat Prod 57:422–425
Fabrice V, Michel G (1990) Enantiomeric purity of scytalone from different fungal sources. Tetrahedron 46:2827–2834
Furukawa A, Arita T, Satoh S, Wakabayashi K, Hayashi S, Matsui Y, Araki K, Kuroha M, Ohsumi J (2010) Discovery of a novel selective PPARc modulator from (−)-Cercosporamide derivatives. Bioorg Med Chem Lett 20:2095–2098
Grove JF (1988) Non-macrocyclic trichothecenes. Nat Prod Rep 5:187–209
Hoffman AM, Mayer SG, Strobel GA, Hess WM, Sovocool GW, Grange AH, Harper JK, Arif AM, Grant DM, Kelley-Swift EG (2008) Purification, identification and activity of phomodione, a furandione from an endophytic Phoma species. Phytochemistry 69:1049–1056
Kiprono PC, Kaberia F, Keriko JM, Karanja JN (2000) The in vitro anti-fungal and anti-bacterial activities of beta-sitosterol from Senecio lyratus (Asteraceae). Z Naturforsch (C) 55:485–488
Liu Z, Jensen PR, Fenical W (2003) A cyclic carbonate and related polyketides from a marine-derived fungus of the genus Phoma. Phytochemistry 64:571–574
Liu L, Li W, Koike K, Zhang S, Nikaido T (2004) New α-tetralonyl glucosides from the fruit of Juglans mandshurica. Chem Pharm Bull 52:566–569
Liu XH, Lu JP, Zhang L, Dong B, Min H, Lin FC (2007) Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryot Cell 6:997–1005
Machida K, Matsuoka E, Kasahara T, Kikuchi M (2005) Studies on the constituents of Juglans species. I. structural determination of (4S)- and (4R)-4-hydroxy-α-tetralone derivatives from the fruit of Juglans mandshurica MAXIM.var. sieboldiana MAKINO. Chem Pharm Bull 53:934–937
Melmed RN, Ishai-Michaeli R, Yagen B (1985) Differential inhibition by T-2 toxin of total protein, DNA and isoprenoid synthesis in the culture macrophage cell line J744. Biochem Pharmacol 34:2809–2812
Michael AC, Mierzwa R, King A, Loebenverg D, Bishop WR, Puar M, Patel M, Coval SJ, Hershenhorn J, Strobel GA (1992) Usnic acid amide, a phytotoxin and antifungal agent from Cercosporidium henningsii. Phytochemistry 31:2999–3001
Mohamed IE, Gross H, Pontius A, Kehraus S, Krick A, Kelter G, Maier A, Fiebig HH, König GM (2009) Epoxyphomalin A and B, prenylated polyketides with potent cytotoxicity from the marine-derived fungus Phoma sp. Org Lett 11:5014–5017
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
O'Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116
Ondeyka JG, Zink DL, Young K, Painter R, Kodali S, Galgoci A, Collado J, Tormo JR, Basilio A, Vicente F, Wang J, Singh SB (2006) Discovery of bacterial fatty acid synthase inhibitors from a Phoma species as antimicrobial agents using a new antisense-based strategy. J Nat Prod 69:377–380
Rosenstein Y, Lafarge-Frayssinet C (1983) Inhibitory effect of Fusarium T2-toxin on lymphoid DNA and protein synthesis. Toxicol Appl Pharmacol 70:283–288
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67:491–502
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Sugawara F, Strobel S, Strobel G (1991) The structure and biological activity of cercosporamide from Cercosporidium henningsii. J Org Chem 56:909–910
Swenson JM, Tenover FC (2005) Results of disk diffusion testing with cefoxitin correlate with presence of mecA in Staphylococcus spp. J Clin Microbiol 43:3818–3823
Talapatra SK, Karmacharya B, De SC (1988) (−)-Regiolone, an α-tetralone from Juglans regia: structure, stereochemistry and conformation. Phytochemistry 27:3929–3932
Tijerino A, Cardoza RE, Moraga J, Malmierca MG, Vicente F, Aleu J, Collado IG, Gutiérrez S, Monte E, Hermos R (2011) Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet Biol 48:285–296
Ueno Y, Nakajima M, Sakai K, Ishii K, Sato N, Shimada N (1973) Comparative toxicology of trichothec mycotoxins: inhibition of protein synthesis in animal cells. J Biochem 74:285–296
Yang X, Strobel G, Stierlea A, Hessb WM, Lee J, Clardy J (1994) A fungal endophyte-tree relationship: Phoma sp. in Taxus wallachiana. Plant Sci 102:1–9
Yano T, Aoyagi A, Kozuma S, Kawamura Y, Tanaka I, Suzuki Y, Takamatsu Y, Takatsu T, Inukai M (2007) Pleofungins, novel inositol phosphorylceramide synthase inhibitors, from Phoma sp. SANK 13899. J Anibiot 60:136–142
Yean HC, Atong M, Chong KP (2009) Lettucenin A and its role against Xanthomonas campestris. J Agr Sci 1:87–93
Zhang X, Geoffroy P, Miesch M, Julien-David D, Raul F, Aoud’e-Werner D, Marchioni E (2005) Gram-scale chromatographic purification of beta-sitosterol synthesis and characterization of beta-sitosteroloxides. Steroids 70:886–895
Zhu H, Ding WJ, Wu R, Weng QJ, Lou JS, Jin RJ, Lu W, Yang B, He QJ (2010) Synergistic anti-cancer activity by the combination of TRAIL/Apo-2L and celastrol. Cancer Invest 28:23–32
Acknowledgments
This work was supported by National Natural Science Foundation of China (30600002 and 30970097) and Special Fund for Agro-scientific Research in the Public Interest (200903052) to Chu-Long Zhang, Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT0943) to Fu-Cheng Lin, and Young Engagement Foundation of Hangzhou Normal University (2010QN21) to Li-Wei Wang.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
NMR data of known compounds (PDF 98 kb)
Rights and permissions
About this article
Cite this article
Wang, LW., Xu, BG., Wang, JY. et al. Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens . Appl Microbiol Biotechnol 93, 1231–1239 (2012). https://doi.org/10.1007/s00253-011-3472-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3472-3