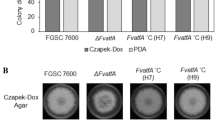
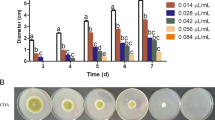
Abstract
Among the various factors correlated with toxin production in fungi, oxidative stress is a crucial one. In relation to this, an important role is played by oxidative stress-related receptors. These receptors can transduce the “oxidative message” to the nucleus and promote a transcriptional change targeted at restoring the correct redox balance in the cell. In Aspergillus parasiticus, the knockout of the ApyapA gene, a homologue of the yeast Yap-1, disables the fungus’s capacity to restore the correct redox balance in the cell. As a consequence, the onset of secondary metabolism and aflatoxins synthesis is triggered. Some clues as to the involvement of oxidative stress in the regulation of ochratoxin A (OTA) synthesis in Aspergillus ochraceus have already been provided by the disruption of the oxylipin-producer AoloxA gene. In this paper, we add further evidence that oxidative stress is also involved in the regulation of OTA biosynthesis in A. ochraceus. In fact, the use of certain oxidants and, especially, the deletion of the yap1-homologue Aoyap1 further emphasize the role played by this stress in controlling metabolic and morphological changes in A. ochraceus.






Similar content being viewed by others
References
Aguirre J, Rios-Momberg MD, Hewitt D, Hansberg W (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol 13:111–118
Aguirre J, Hansberg W, Navarro R (2006) Fungal responses to reactive oxygen species. Med Mycol 44:101–107
Banerjee D, Madhusoodanan UK, Sharanabasappa M, Ghosh S, Jacob J (2003) Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenylphosphine. Clin Chim Acta 337:147–152
Calvo AM, Wilson RA, Bok JW, Keller NP (2002) Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev 66:447–459
Castoria R, Caputo L, De Curtis F, De Cicco V (2003) Resistance of post-harvest biocontrol yeasts to oxidative stress: a possible new mechanism of action. Phytopathol 93:564–572
Cheeke PR (1998) Natural toxicants in feeds, forages and poisonous plants. Interstate, Danville
Commission of the European Community (2006) Regulation (EC) no. 1881/2006 of 19 December 2006: setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L 364:5–24
Creppy EE, Chakor K, Fisher MJ, Dirheimer G (1990) The mycotoxin ochratoxin A is a substrate for phenylalanine hydroxylase in isolated rat hepatocytes and in vivo. Arch Toxicol 64:279–284
Eskola M, Kokkonen M, Rizzo A (2002) Application of manual and automated systems for purification of ochratoxin A and zearalenone in cereals with immunoaffinity columns. J Agric Food Chem 50:41–47
Estruch F (2000) Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol Rev 24:469–486
Fisher R (2008) Sex and poison in the dark. Science 320:1430–1431
Fox ME, Howlett BJ (2008) Secondary metabolism: regulation and role in fungal biology. Curr Op Microbiol 11:481–487
Georgiou CD, Patsoukis N, Papapostolou I, Zervoudakis G (2006) Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr Comp Biol 46:691–712
Goldstein J, Newbury D, Joy D, Lyman C, Echlin P, Lifshin E, Sawyer L, Michael J (2003) Scanning electron microscopy and x-ray microanalysis, 3rd edn. Springer, New York, p 689
Halliwell B, Gutteridge JMC (2007) Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, New York, pp 187–267
IARC (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines, and mycotoxins. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, vol 56. International Agency for Research on Cancer, Lyon, p 599
Jayashree T, Subramanyam C (2000) Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Rad Biol Med 29:981–985
Kim JH, Campbell BC, Yu J, Mahoney N, Chan KL, Molyneux RJ, Bhatnagar D, Cleveland TE (2004) Examination of fungal stress response genes using Saccharomyces cerevisiae as a model system: targeting genes affecting aflatoxin biosynthesis by Aspergillus flavus Link. Applied Microbiol Biotechnol 67:807–815
Lledias F, Rangel P, Hansberg W (1999) Singlet oxygen is part of a hyperoxidant state generated during spore germination. Free Rad Biol Med 26:1396–1404
Molina L, Kahmann R (2007) An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell 19:2293–22309
Moye-Rowley WS (2003) Regulation of transcriptional response to oxidative stress in fungi: similarities and differences. Eukaryot Cell 2:381–389
Narasaiah KW, Sashidhar RB, Subramanyam C (2006) Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathol 162:179–189
O’Callaghan J, Caddick MX, Dobson ADW (2003) A polyketide synthase gene required for ochratoxin A biosynthesis in Aspergillus ochraceus. Microbiol 149:3485–3491
O’Callaghan J, Stapleton PC, Dobson ADW (2006) Ochratoxin A biosynthetic genes in Aspergillus ochraceus are differentially regulated by pH and nutritional stimuli. Fungal Genet Biol 43:213–221
Pallardò FV, Markovic J, Garcìa JL, Viña J (2009) Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspects Med 30:77–85
Pardo E, Marin S, Ramos A, Sanchis V (2004) Occurrence of ochratoxigenic fungi and ochratoxin A in green coffee from different origins. Food Sci Technol Int 10(1):45–49
Passi S, Fanelli C, Fabbri AA, Finotti E, Panfili G, Nazzarro-Porro M (1985) Effect of halomethanes on aflatoxin induction in cultures of Aspergillus parasiticus. J Gen Microbiol 131:687–691
Payne GA, Hagler WM (1983) Effect of specific aminoacids on growth and aflatoxin by Aspergillus parasiticus and A. flavus in defined media. Appl Environ Microbiol 46:805–812
Ponts N, Pinson-Gadais L, Verdal-Bonnin MN, Barreau C, Richard-Forget F (2006) Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol Lett 258:102–107
Reverberi M, Fabbri AA, Zjalic S, Ricelli A, Punelli F, Fanelli C (2005) Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl Microbiol Biotechnol 69:207–215
Reverberi M, Zjalic S, Ricelli A, Punelli F, Camera E, Fabbri C, Picardo M, Fanelli C, Fabbri AA (2008) Modulation of antioxidants defence in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for ApyapA gene. Eukaryot Cell 7(6):988–1000
Reverberi M, Punelli F, Scarpari M, Camera E, Zjalic S, Ricelli A, Fanelli C, Fabbri AA (2010) Lipoperoxidation affects ochratoxin A biosynthesis in Aspergillus ochraceus and its interaction with wheat seeds. Appl Microbiol Biotechnol 85:1935–1946
Roze LV, Chanda A, Wee J, Awad D, Linz JE (2011) Stress-related transcription factor AtfB integrates secondary metabolism with oxidative stress response in Aspergilli. J Biol Chem 286(40):35137–35148
Solfrizzo M, Avantaggiato G, Visconti A (1998) Use of various cleanup procedures for the analysis of ochratoxin A in cereals. J Chromat 815:67–73
Stoev S (1998) The role of ochratoxin A as a possible cause of Balkan endemic nephropathy and its risk evaluation. Vet Hum Toxicol 40:352–360
Tolaini V, Zjalic S, Reverberi M, Fanelli C, Fabbri AA, Ricelli A (2010) Lentinula edodes enhances the biocontrol activity of Cryptococcus laurentii against Penicillium expansum contamination and patulin production in apple fruits. Int J Food Microbiol 138:243–248
Van der Merwe KJ, Steyne KJ, Fourie L, Scott DB, Theron JJ (1965) Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 205:1112–1113
WHO (2001) Safety evaluation of certain mycotoxins in food. Fifty sixth report of the joint FAO/WHO expert committee on food additives. WHO Food Additives Series 47. World Health Organization, Geneva, p 700
Wilkinson JR, Yu J, Bland JM, Nierman WC, Bhatnagar D, Cleveland TE (2007) Amino acid supplementation reveals differential regulation of aflatoxin biosynthesis in Aspergillus flavus NRRL 3357 and Aspergillus parasiticus SRRC 143. Appl Microbiol Biotechnol 74(6):1308–1319
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reverberi, M., Gazzetti, K., Punelli, F. et al. Aoyap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus . Appl Microbiol Biotechnol 95, 1293–1304 (2012). https://doi.org/10.1007/s00253-012-3985-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3985-4