Abstract
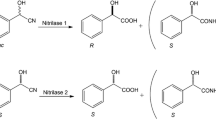
Alcaligenes sp. MTCC 10675 has been isolated from soil sample using enrichment method and has nitrilase catalytic system which is highly specific for the hydrolysis of arylaliphatic nitriles. Optimization of culture conditions using response surface methodology and inducer-mediated approach enhanced arylacetonitrilase production significantly (2.4-fold). Isobutyronitrile acted as an effective inducer for the induction of arylacetonitrilase, and it is highly specific for arylacetonitriles (phenyl acetonitrile and mandelonitrile). Arylacetonitrilase has no effect on its relative velocity (V r) up to 20 mM substrate (mandelonitrile) concentration and at 30 mM mandelonitrile, 23.4 % degree of inhibition (I d) was recorded. Half life of arylacetonitrilase of Alcaligenes sp. MTCC 10675 was 27.5 h at 25 °C. Hg2+, Ag+, Pb3+, and Co2+ were strong inhibitor of arylacetonitrilase activity which resulted into 100 %, 91 %, 84 %, and 83 % inhibition, respectively. Polar protic solvent (dichloromethane, dimethylsulphooxide, and n-butanol) reduce arylacetonitrilase activity up to 80–94 % at 10 % concentration. Alcaligenes sp. MTCC 10675 has higher biocatalytic activity, i.e., 3.9 gg-1 dcw, which is highest in comparison to till reported organism. Arylacetonitrilase-mediated hydrolysis of racemic mandelonitrile resulted into R-(-) mandelic acid with 99.0 % enantiomeric excess (e.e.)
Similar content being viewed by others
Introduction
Mandelic acid is a type of alpha-hydroxy acid and used as chiral synthon for the synthesis of various pharmaceutical intermediate, i.e., penicillin, cephalosporin, antiobesity, and antitumor agent (Banerjee et al. 2006; He et al. 2010). There are different chemical and biotechnological routes available for the mandelic acid production. Biotechnologically mandelic acid can be synthesized by reduction of benzoylformic acid or hydrolysis of racemic mandelonitrile (Yamazaki and Maeda 1986; Yamamoto and Komatsu 1991; Kaul et al. 2004; He et al. 2010; Jain et al. 2012). Arylacetonitrilase is the nitrilase superfamily enzyme, and one important application of this enzyme is the conversion of mandelonitrile into mandelic acid (Yamamoto and Komatsu 1991; He et al. 2010). The arylacetonitrilase-mediated pathways for the hydrolysis of mandelonitrile has advantage over other routes, because it requires no cofactor, and inexpensive mandelonitrile acts as the starting material (Gong et al. 2012). Most of the organisms used in the synthesis of mandelic acid have lower biocatalytic activity, specificity, and product tolerance, so immobilization and other cloning strategies are used which are cost-effective (Rustler and Stolz 2007; Banerjee et al. 2009; He et al. 2010). In order to develop an economic process for the synthesis of mandelic acid, there is a need to isolate microorganisms having higher biocatalytic activity and product tolerance The objective of present study was to isolate bacterial strain having capacity to hydrolyze arylacetonitriles (mandelonitrile and phenylacetonitriles) and to optimize the culture conditions using response surface methodology for enhanced production of arylacetonitrilase. Free cells of Alcaligenes sp. MTCC 10675 has been used for the hydrolysis of racemic mandelonitrile into (R)-mandelic acid at 1 l fed batch scale and reused to harness their full hydrolytic potential.
Materials and methods
Chemicals
Mandelonitrile and isobutyronitrile were purchased from Alfa Aesar, Johnson Matthey. Different media components were obtained from Hi-Media (Mumbai, India). All other chemicals used were of analytical grade and procured from standard companies.
Isolation of bacterial strain
Soil and water samples were collected from different places of Himachal Pradesh, India. The nitrile transforming bacteria were isolated from these samples by enrichment culture technique, using medium supplemented with filter sterile 0.4 % v/v isobutyronitrile (Bhalla et al. 1992). The pure line culture of bacterial isolates were prepared and maintained on nutrient agar slant at 4 °C.
Screening of bacterial isolates for mandelonitrile-hydrolyzing activity
Nutrient broth (pH 7.0) was used as seed medium and the culture was incubated at 30 °C for about 18 h in a rotary shaker (150 rpm). Subsequently 2 % inoculum was transferred into 250 ml Erlenmeyer flask containing 50 ml of nutrient broth as production medium. Isobutyronitrile (0.4 %) was used as inducer for the induction of arylacetonitrilase activity. These flasks were incubated at 30 °C for 24 h at 150 rpm in an incubator shaker. After 24 h cells, were harvested by centrifugation (6000 × g for 10 min at 4 °C), washed with potassium phosphate buffer (0.1 M pH 7.0) and suspended in the same buffer. The cell suspension containing resting cells was used as biocatalyst for the transformation of mandelonitrile into mandelic acid. The hyper active bacterial isolate S31 was identified as Alcaligenes sp. at the Microbial Type Culture Collection & Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh, India and the accession number assigned to it as MTCC 10675.
Arylacetonitrilase assay
The reaction mixture (1 ml) for arylacetonitrilase assay of Alcaligenes sp. MTCC 10675 contained 880 μl of 0.1 M potassium phosphate buffer (pH 7.0), 100 μl resting cell (10 mg dcw ml-1), and 20 μl (0.5 M) mandelonitrile. The reaction mixture was incubated for 1 h at 30 °C and 1 ml of 0.1 M HCl was added to stop the reaction followed by centrifugation at 6,000 × g for 10 min to remove the free cells. The amount of ammonia released in the reaction mixture was estimated in the supernatant by using the phenate-hypochlorite method (Fawcett and Scott 1969). One unit of enzyme activity was defined as the amount of enzyme that produces 1 μmole of mandelic acid or ammonia per min under standard assay condition.
HPLC analysis of the reaction mixture was performed using Oyster ODS3 (150 × 4.6 mm, 5.0 μm) column and acetonitrile: water (65:35) used as mobile phase at 210 nm. The retention time for mandelic acid and mandelonitrile were 1.17 and 2.4 min, respectively. The optical purity of mandelic acid was determined by using HPLC equipped with Chiral-cel ODH column (250 × 0.46 mm, 5 μm, Daicel Chemical Industry, USA) and elution was performed with hexane/isopropanol/trifluoroacetic acid (90:10:0.2, v/v/v) at flow rate of 1 ml min-1 . The retention time for S-(+) mandelic acid and R-(-)- mandelic acid was 7.2 and 8.9 min at A 210 nm
Optimization of culture conditions
Medium optimization
Alcaligenes sp. MTCC 10675 was cultivated in different media M1, M2, M3, M4, M5 and M6 (pH 7.0), which have been earlier reported for the growth and production of nitrile-hydrolyzing enzymes in various organisms (Table 1) (Bhatia et al. 2013). The arylacetonitrilase activity of the cells grown in various media was assayed as mentioned above.
Carbon and nitrogen source
Different types of organic and inorganic source of carbon (1.0 %) and nitrogen (1.0 %) were studied for the growth and arylacetonitrilase production by Alcaligenes sp. MTCC 10675.
Inducer
Various inducers (aliphatic, aromatic and heterocyclic nitrile) were added at a concentration of 40 mM in the production medium to select an appropriate inducer for the induction of arylacetonitrilase activity in Alcaligenes sp. MTCC 10675.
Factorial design
Plackett–Burman design
Effect of eight independent factor glycerol (0.1 %–2 %), peptone (0.1 %–0.5 %), yeast extract (0.1 %–0.5 %), malt extract (0.1 %–0.5 %), pH (4.0–10), temperature (20–55), inducer (0–360 μl/100 ml) and inoculum volume (0.5 %–5 %) on Alcaligenes sp. MTCC 10675 growth and arylacetonitrilase activity were screened by using Plackett–Burman design of Design Expert software 8.0.4. Experiments were performed at various combination of high and low level of variables and analyzed for their effect on response of process. Pareto chart was constructed to find out the positive variable which effect Alcaligenes sp. MTCC 10675 arylacetonitrilase activity and growth.
Central composite design
Central composite design CCD experiment was designed for the optimization of optimum value of inducer; peptone, yeast extract and pH which showed positive effect on Alcaligenes sp. MTCC 10675 growth and mandelonitrile-hydrolyzing activity. Second-order polynomial coefficient were calculated and analyzed by using Design Expert software 8.0.4.
Validation of statistical model
The statistical model was validated for the production of arylacetonitrilase by performing experiment at shake flask under predicted set of conditions.
Time course of inducer feeding
Several sets of experiment were carried out to increase the arylacetonitrilase production by Alcaligenes sp. MTCC 10675. Biomass production and arylacetonitrilase activity of Alcaligenes sp. MTCC 10675 were monitored up to 42 h. In control 1, no isobutyronitrile was added, control 2 (without additional carbon and inducer); in set 1, 2, 3 and 4 where 20 mM, 40 mM, 60 mM and 80 mM inducer was added, respectively, at 0 h. In set 5 (40 mM after 6 h) and set 6 (60 mM after 6 h) while in set 7 (40 mM at 0 and 12 h) and set 8 (60 mM at 0 and 12 h) isobutyronitrile was added.
Optimization of reaction conditions
The reactions for assay of mandelonitrile-hydrolyzing activity were carried out for 60 min using various buffer systems, i.e., citrate buffer pH (4.0–6.0), potassium phosphate buffer (6.0–8.0), sodium phosphate buffer (6.0–8.0), borate buffer (7.0–9.0) and carbonate buffer (9.0–10.0) of 0.1 M and reaction temperature (20–60 °C). The effects of substrate concentration (0.01–0.09 M mandelonitrile), temperature (25, 35, 45, and 55 °C), metals ions and organic solvents on the arylacetonitrilase activity of Alcaligenes sp. MTCC 10675 were studied.
Bioprocess development for mandelic acid production
Time course of mandelonitrile conversion
Substrate concentration and arylacetonitrilase activity were varied as 10 mM: 2 U ml-1, 20 mM: 4 U ml-1, 30 mM: 6 U ml-1, 40 mM: 8 U ml-1, 50 mM: 10 U ml-1, and 60 mM: 12 U ml-1 in the reaction, and samples were withdrawn at every 20 for 100 min and analyzed for 100 % conversion of substrate to product in shortest possible incubation time and to study the conversion rate with increasing substrate and biocatalyst (resting cell) concentration.
Fed batch reaction at 40 ml scale (40 mM mandelonitrile per feed)
Fed batch reaction at 40 ml scale was performed by feeding 40 mM substrate at 60 min interval and resting cells equal to 8 U ml-1 were used, thus six feedings were made and a 240 mM substrate was added in the reaction in 6 h.
Fed batch reaction at 40 ml scale (10 mM mandelonitrile per feed)
The arylacetonitrilase activity declined in presence of high substrate concentration as analyzed in the above experiment. A feeding of 10 mM mandelonitrile at an interval of 20 min was made and total 200 mM mandelonitrile was fed in 20 feedings.
Fed batch reaction at 1 l scale (10 mM mandelonitrile per feed)
The reaction volume for the conversion of mandelonitrile was scaled up to 1 l. This reaction was carried out in a 1.5 l fermenter (BioFlow C-32 New Brunswick Scientific, USA). The substrate corresponding to 10 mM was fed in every 20 min. A total of 150 mM of substrate and resting cells equal to 8,000 U of arylacetonitrilase were used in the reaction mixture in 5 h. The temperature of the reaction mixture was maintained at 30 °C, while the impeller speed was set to 200 rpm.
Results
Medium optimization
Alcaligenes sp. MTCC 10675 was cultured in different media and arylacetonitrilase activity was maximum (0.10 U mg-1 dcw) in M4 medium with biomass production of 11 mg dcw ml-1. Alcaligenes sp. MTCC 10675 exhibited good biomass production in all the media except M5, but production of arylacetonitrilase activity was low in M1, M2, M3, and M6 media (Table 1). In medium M5 (minimal medium), Alcaligenes sp. MTCC 10675 showed poor biomass production (5.0 mg dcw ml-1) and activity (0.02 U mg-1 dcw).
Carbon source
A number of carbon sources were tested to enhance the growth and production of arylacetonitrilase activity of Alcaligenes sp. MTCC 10675. Control 1 (without additional carbon) showed maximum activity of 0.12 U mg-1 dcw with 10.1 mg dcw ml-1 biomass production. In the presence of fructose, galactose, glucose, and sodium acetate, Alcaligenes sp. MTCC 10675 has good biomass, but arylacetonitrilase activity was lower than control 1. Addition of glycerol, mannitol, sucrose, and sodium citrate in medium resulted into higher production of arylacetonitrilase, but biomass production was less as compared to control 1. Control 2 (without additional carbon and inducer) resulted in very little growth (4.2 mg dcw ml-1) and activity (0.02 U mg-1 dcw). In terms of arylacetonitrilase activity and biomass production, medium with carbon control was selected.
Selection of inducer
Various aliphatic, aromatic, heterocyclic, and hydroxynitriles were used as inducer for the induction of arylacetonitrilase activity in Alcaligenes sp. MTCC 10675. Short-chain aliphatic nitriles, i.e., isobutyronitrile (0.14 U mg-1 dcw), adiponitrile (0.13 U mg-1 dcw), butyronitrile (0.13 U mg-1 dcw), propionitrile (0.09 U mg-1 dcw), and heterocyclic nitrile (2-cyanopyridine 0.12 U mg-1 dcw) acted as a good inducer for the induction of arylacetonitrilase activity in Alcaligenes sp. MTCC 10675. In the presence of hydroxynitrile, dinitriles, aromatic nitrile and arylaliphatic nitrile in medium, Alcaligenes sp. MTCC 10675 did not grow, which exhibited that nitriles acted as source of carbon. The Alcaligenes sp. MTCC 10675 showed 10.2 mg dcw ml-1 biomass production and arylacetonitrilase activity (0.14 U mg-1 dcw) in the presence of isobutyronitrile as inducer in the medium.
Substrate specificity of Alcaligenes sp. MTCC 10675
Alcaligenes sp. MTCC 10675 was highly specific toward the arylacetonitrile and it exhibited 0.40 and 0.15 U mg-1 dcw arylacetonitrilase activity to words phenylacetonitrile and mandelonitrile, respectively. Arylacetonitrilase of Alcaligenes sp. MTCC 10675 has lower affinity toward aliphatic, aromatic, and heterocyclic nitrile. Alcaligenes sp. MTCC 10675 showed maximum amide-hydrolyzing activity against butyramide (0.182 U mg-1 dcw) followed by propionamide (0.139 U mg-1 dcw) and acetamide (0.126 U mg-1 dcw), and no activity was recorded against aromatic, heterocyclic, and hydroxyamide (Table 2). The reaction mixture containing various nitriles was also analyzed for amide formation during the hydrolysis of nitriles, and there was no amide formation was detected.
Factorial design
Plackett–Burman design
Factorial design was used for the screening of various medium components for biomass and arylacetonitrilase production by Alcaligenes sp. MTCC 10675. Medium optimized by using single variable at a time was further used for designing factorial design. Optimized medium components (glycerol, peptone, yeast extract, malt extract, inducer, and inoculums) and other physiochemical parameters (pH, temperature) were screened for their effect on arylacetonitrilase activity using Plackett–Burman design (Table 3).
A total of 12 experiments were performed, and 0 to 0.194 U mg-1 dcw variations in activity was observed. To find out the order of significance of variable on arylacetonitrilase production Plackett–Burman design with eight variables was further used to construct a Pareto chart (Fig. 1) on the basis of responses of different independent variables. Peptone, yeast extract, inducer, and pH were analyzed as positive factor in comparison to temperature, glycerol, malt extract, and inoculum, which showed negative effect on arylacetonitrilase activity. Inducer and peptone concentration have stronger influence on arylacetonitrilase activity as compared to yeast extract and pH.
ANOVA analysis of Plackett–Burman results was performed and a first-order polynomial equation was derived to explain the effect of various variables on arylacetonitrilase production.
R 1 = +0.075 + 2.583E-003*A-9.417E-003*C + 0.018*D + 8.917E-003 + 0.054*G4.917E-003*H
This equation showed the magnitude of effect of different independent variables. R 1 is the activity (U mg-1 dcw) and A, C, D, E, G, and H are the coded value of different variable, i.e., pH, glycerol, peptone, yeast extract, inducer, and inoculum. Effective levels of various component (pH, peptone, yeast extract, and inducer) were further investigated by designing central composite design.
CCD and response surface analysis
Based on the results of Plackett–Burman design, the effect of variable pH, peptone, yeast extract and inducer on the production of arylacetonitrilase were examined at five levels. A set of 30 experiments were carried out as listed in Table 4.
The following regression equation was derived after the analysis of variance.
R1 = +0.23 + 2.125E-003*A + 7.208E-003*B + 3.208E-003*C + 0.035*D + 1.875E-004*A*B-1.813E-003*A*C + 8.125E-004*A*D-2.562E-003*B*C + 2.563E-003*B*D-4.375E-004*C*D-0.063*A2-0.017*B2-0.020*C2-0.062*D2
Lack of fit test for CCD model
ANOVA analysis of the central composite design (CCD) result was performed and four process order was suggested by Design Expert 8.0.4. Quadratic process order proved best and used for further analysis due to low standard deviation (0.042), high R-squared value (0.894) and low press value (0.15) for further analysis.
Analysis of the variance of the model
The analysis of the quadratic regression model suggested that the model was very significant as correlation coefficient (0.894) closer to 1 denotes better correlation between the observed and predicted responses. Lower value of coefficient of variance (CV 12.34) denotes that the experiment performed was reliable. Three-dimensional plots were generated for regression analysis of CCD design of pairwise combination of three factors for arylacetonitrilase production. The effects of the independent variables and combined effects of each independent variable upon the response variable were described by plotting 3d graphs (Fig. 2a, b, c, d, e and f).
Validation of model
The maximum production of arylacetonitrilase was obtained by performing experiment closely related to the 0.239 U mg-1 dcw, predicted value calculated by ANOVA analysis. Perturbation plot (Fig. 3) showed the optimum value for variables pH 7.0, peptone 0.55, yeast extract 0.55, inducer 180 μl (40 mM) per 100 ml. The model was validated by performing the experiment under optimum conditions, which resulted into 0.221 U mg-1 dcw activity and proved the validity of the model. Thus 1.6-fold increase in arylacetonitrilase activity was recorded.
Hyperinduction of arylacetonitrilase of Alcaligenes sp. MTCC 10675
Several experiments were carried out to increase the production of arylacetonitrilase in Alcaligenes sp. MTCC 10675. Biomass and arylacetonitrile production by Alcaligenes sp. MTCC 10675 were monitored up to 42 h. In control 1, where no isobutyronitrile was added, Alcaligenes sp. MTCC 10675 entered in stationary phase of growth (3.7 mg dcw ml-1) after 18 h (Fig 4a), and no arylacetonitrilase activity was observed (Fig. 4b). Specific activity goes on increasing up to 24 h in set 1 experiment, where 20 mM isobutyronitrile was used as inducer along with biomass, and it took 18 h to reach the stationary phase (6.9 mg dcw ml-1) by Alcaligenes sp. MTCC 10675. In set 2, set 3, and set 4, respectively, 40, 60, and 80 mM inducer was added and Alcaligenes sp. MTCC 10675 entered in stationary phase (2.5 mg dcw ml-1) in 24 h with 0.25 U mg-1 dcw activity, while in set 3 and set 4, organism grew continuously, but there was no significant increase in enzyme production (Fig. 4b). In set 5 and set 6, addition of inducer after 6 h resulted in slow growth in starting without any increase in activity, and after addition of inducer, there was increase in biomass as well as enzyme activity. In set 5, maximum activity 0.23 U mg-1 dcw was achieved in 24 h, and in set 6, 0.33 U mg-1 dcw activity was recorded. Two time addition of inducer in set 7 and set 8 increased biomass (Fig. 4c), but there is no increase in activity corresponding to biomass (Fig. 4d). Experiment set 6 (60 mM after 6 h) proved to be the best inducer mediation approach for the production of arylacetonitrilase activity (0.33 U mg-1 dcw).
Optimization of reaction conditions
Buffer system
Enzyme assay was carried out in different buffer systems at various pH (4.0–10.0) to find out the optimum pH for arylacetonitrilase activity. Maximum arylacetonitrilase activity (0.315 U mg-1 dcw) was observed in 0.1 M potassium phosphate buffer (6.0 pH). Increase in arylacetonitrilase activity was recorded in citrate buffer up to 5.0 pH. Arylacetonitrilase activity decreased at higher pH buffer, i.e., borate (0.277 U mg-1 dcw, pH 8.5) and carbonates buffer (0.199 U mg-1 dcw, pH 9.0). Increase in arylacetonitrilase activity and enantioselectivity was observed with the increase of pH, and (R)-mandelic acid was obtained with 99.0 % enantiomeric excess (e.e.) at pH 6.0.
Temperature
The reaction of arylacetonitrilase was carried out at different temperature (10–75 °C) for 60 min. Arylacetonitrilase activity increased with the increase of temperature and optimum temperature for arylacetonitrilase assay of Alcaligenes sp. MTCC 10675 (0.36 U mg-1 dcw) was 45 °C. Arylacetonitrilase activity was stable at 30–55 °C. Above 55 °C, there was a rapid decrease in arylacetonitrilase activity. Arylacetonitrilase of Alcaligenes sp. MTCC 10675 was active in the temperature range of 40–50 °C.
Substrate concentration
The effect of substrate (mandelonitrile) concentration on the activity of arylacetonitrilase was studied, and maximum activity (0.46 U mg-1 dcw) was observed at 20 mM. Increase in activity was recorded up to 20 mM, after which there was no increase in activity and a sharp decrease in activity recorded with the increase in concentration of mandelonitrile. Increase in substrate concentration from 20 to 40 mM resulted in 61 % loss in this enzyme activity. A Lineweaver–Burk plot (1/V versus 1/S) of the velocity of arylacetonitrilase reaction at different substrate concentration showed that above 20 mM concentration of mandelonitrile, arylacetonitrilase did not follow Michelis–Menten kinetics. Arylacetonitrilase encountered substrate inhibition; V max and K m value for the arylacetonitrilase were calculated from graph as 1 μmole mg-1 min-1 and 40 mM, respectively. The relative velocity (V r) and degree of inhibition (I d) of arylacetonitrilase was calculated, at 20 mM concentration, V r and I d were 100 % and 0 %, respectively. This meant that enzyme has not experienced substrate inhibition up to 20 mM mandelonitrile concentration. Above this concentration, arylacetonitrilase faced substrate inhibition, and at 30 mM mandelonitrile, V r and I d were 76.6 % and 23.4 %, respectively. At 90 mM mandelonitrile, arylacetonitrilase activity was completely inhibited and 93 % I d was recorded.
Cell concentration
Effect of resting cells concentration on arylacetonitrilase activity was studied for 20 mM mandelonitrile and maximum activity (0.394 U mg-1 dcw) was observed at 0.8 mg dcw ml-1. There was continuous increase in arylacetonitrilase activity up to 0.8 mg dcw ml-1 cell concentration after which cell and substrate attained a saturation stage, and no increase in activity was observed.
Reaction time
For the conversion of mandelonitrile into mandelic acid, the time course of arylacetonitrilase activity was studied up to 120 min. Maximum arylacetonitrilase activity was observed after 70 min (0.34 U mg-1 dcw) with complete conversion of mandelonitrile. Initially as the incubation time increased, mandelic acid production also increased up to 90 min; further increase in reaction time did not lead to any increase in product formation. Thermal and operational stability of Alcaligenes sp. MTCC 10675 arylacetonitrilase was studied at different temperature 25–55 °C. Arylacetonitrilase of Alcaligenes sp. MTCC 10675 has half life of 27.5, 12.9, 3.9, and 2.8 h at 25, 35, 45, and 55 °C, respectively. With the increase of temperature, decrease in arylacetonitrilase activity and stability was observed, at 45 and 55 °C arylacetonitrilase of Alcaligenes sp. MTCC 10675 has lost its activity and only 0.102 U mg-1 dcw and 0.013 U mg-1 dcw activities were detected after 6 h.
Effect of metal ions
The arylacetonitrilase activity of Alcaligenes sp. MTCC 10675 was evaluated in the presence of various metals ions. Hg2+, Ag+, Pb3+, and Co2+ were strong inhibitor of arylacetonitrilase activity which resulted into 100 %, 91 %, 84 %, and 83 % inhibition, respectively. Cs2+, K+, EDTA, DTT, and urea have very little effect on mandelonitrile-hydrolyzing activity, while Cu2+ , Mg2+, and Zn2+ caused up to 50 % decrease in arylacetonitrilase activity. The DTT, urea, and chelating agent EDTA showed no significant effect on the activity of arylacetonitrilase.
Effect of solvents
Nonpolar organic solvents have affected the arylacetonitrilase activity. Diethyl ether reduced 30 % of arylacetonitrilase activity at 30 % organic solvent while benzene, chloroform, and toluene decreased half of activity at 10 % concentration (v/v) in reaction. Among polar aprotic solvents, dichloromethane and dimethylsulphooxide are most effective solvents, which results into more than 80 % reduction in activity at 10 % concentration. Addition of polar protic solvents resulted in drastic decrease in arylacetonitrilase activity, e.g., at 10 % n-butanol, there was 94 % loss of arylacetonitrilase activity. Ethanol and methanol at 10 % concentration did not cause much loss to the enzyme activity, but at concentration of more than 20 %, these solvent caused substantial loss to the activity of arylacetonitrilase.
Bioprocess development
Time course of mandelonitrile conversion
The effect of increasing concentration of arylacetonitrilase with respect to the substrate concentrations was studied, the reaction mixture containing 8 U ml-1 arylacetonitrilase activity and 40 mM of mandelonitrile showed highest conversion rate, and at the same time, higher yield of mandelic acid was also achieved in 1 h 20 min. The rate of formation of mandelic acid was more or less similar in the presence of 10 and 40 mM mandelonitrile. The high concentration of substrate (50 mM) had inhibitory effect on formation of mandelic acid, although the enzyme concentration in the reaction was proportionally increased (Fig. 5a)
Fed batch reaction at 40 ml scale (40 mM mandelonitrile)
Fed batch reaction at 40 ml scale, containing 8 U ml-1 arylacetonitrilase activity resting cells led to 100 % conversion to product in 1 h 20 min. The rate of mandelic acid production declined sharply on further feeding of mandelonitrile. This resulted in the accumulation of 133 mM of mandelic acid and 147 mM residual mandelonitrile after seven feedings.
Fed batch reaction at 40 ml scale (10 mM mandelonitrile)
The arylacetonitrilase activity declined in the presence of high substrate concentration as described in the above experiment. A feeding of 10 mM at an interval of 20 min was made. There was no inhibition of arylacetonitrilase activity till ten feeds, and maximum substrate was converted to mandelic acid. After 10th feed up to 20th feed, the rate of mandelonitrile conversion went on declining continuously (Fig. 5b). This resulted in the accumulation of 43 mM residual mandelonitrile after 20 feeds and only 157 mM mandelic acid was formed. The HPLC analysis showed that R-(-)-mandelic acid with e.e. of 99.0 % was the main product without production of any side product. Thus arylacetonitrilase was the only enzyme that led to conversion of mandelonitrile into mandelic acid.
Fed batch reaction at 1 l scale (10 mM mandelonitrile/20 min) and reusability of cell
Fed batch reaction at 1 l scale resulted into accumulation of 130 mM of mandelic acid. Recycled resting cells from the above reaction of 1 l scale, reused in the next batch of reaction. Arylacetonitrilase activity of Alcaligenes sp. MTCC 10675 goes on decreasing in each cycle due to cell damage and thermal instability, but corresponding decrease in substrate concentration during each feed led to complete conversion of mandelonitrile into mandelic acid. Mandelic acid was recovered from the reaction by using diethyl ether as solvent. Lypholization and vacuum drying of reaction supernatant resulted in the formation of white precipitates of mandelic acid. The filtration and drying of the precipitate finally led to recovery of mandelic acid with 0.78 gl-1 h-1 volumetric productivity and 3.9 gg-1 dcw catalytic productivity in fed batch reaction. Racemic mandelonitrile was hydrolyzed into R-(-)-mandelic acid with 99.0 e.e. and 85 % yield was obtained. Thus a loss of 15 % of the mandelic acid during downstream processing was observed.
Discussion
A number of bacterial strains have been isolated, immobilized, and cloned for the production of mandelic acid using mandelonitrile as substrate, i.e., Alcaligenes faecalis ATCC 8750, Pseudomonas putida MTCC 5110, A. faecalis ECU0401, Pseudomonas fluorescens EBC191, and A. faecalis CCTCC M 208168 (Yamamoto et al. 1992; Banerjee et al. 2009; He et al. 2010; Rustler and Stolz 2007; Xue et al. 2010). Alcaligenes sp. MTCC 10675 showed poor biomass production (5.0 mg dcw ml-1) and arylacetonitrilase activity (0.02 U mg-1 dcw) in medium M5 (minimal medium like other organism Rhodococcus rhodochrous J1 and R. rhodochrous K22 (Nagasawa et al. 1988; Kobayashi et al. 1991), while in medium M4 supplemented with peptone and yeast extract, good biomass production and arylacetonitrilase activity was achieved (He et al. 2010). Addition of carbon source in the production medium resulted into catabolite repression and reduction in arylacetonitrilase activity, so a medium without carbon source was selected for Alcaligenes sp. MTCC 10675 production (Banerjee et al. 2006). The Alcaligenes sp. MTCC 10675 showed 10.2 mg dcw ml-1 biomass production and 0.14 U mg-1 dcw arylacetonitrilase activity in the presence of isobutyronitrile as inducer in the medium. This observation is similar to earlier reports on the production of arylacetonitrilase activity in A. faecalis ATCC 8750 and A. faecalis CCTCC M 208168 (Yamamoto and Komatsu 1991; Xue et al. 2010). Arylacetonitrilase of Alcaligenes sp. MTCC 10675 has lower affinity toward aliphatic, aromatic, and heterocyclic nitrile and more substrate specificity to words the arylacetonitriles like the P. putida EBC 191 and A. faecalis ECU0401 having arylacetonitrile (phenylacetonitriles > mandelonitrile)-hydrolyzing activity (He et al. 2010; Rustler and Stolz 2007). The activity that hydrolyze various nitriles into their corresponding acids was detected but no amide formation was recorded which demonstrated that this microorganism has arylacetonitrilase activity but not a nitrile hydratase activity (Yamamoto et al. 1992; He et al. 2010). Alcaligenes sp. MTCC 10675 showed maximum amide-hydrolyzing activity against short-chain aliphatic amide but there was no amidase activity was recorded against aromatic, heterocyclic and arylaliphatic amides similar to the amidase of Rhodococcus sp. Strain R312 (Fournand et al. 1998). There was 1.5-fold (49 %) increase in arylacetonitrilase activity for mandelonitrile by using inducer feeding approach, while 1.1-fold increases in nitrilase activity of P. putida was reported (Banerjee et al. 2006). Alcaligenes sp. MTCC 10675 has maximum arylacetonitrilase activity at pH 6–7 as already reported for the arylacetonitrilase of P. putida and P. fluorescens EBC191 (Banerjee et al. 2006; Sosedov et al. 2010). Enantioselectivity of arylacetonitrilase of Alcaligenes sp. MTCC 10675 increased with the increase of pH, because alkine pH accelerate the racemization of unreacted (S)-mandelonitrile and resulted into mandelic acid with high e.e. (Zhang et al. 2011). Arylacetonitrilase of Alcaligenes sp. MTCC 10675 was active in the temperature range of 40–50 °C, similar to the arylacetonitrilase of A. faecalis JM3, A. faecalis ATCC 8750, Bradyrhizobium japonicum USDA110, Aspergillus niger K10, and Fusarium solani O1 (Nagasawa et al. 2010; Yamamoto et al. 1992; Zhu et al. 2008; Kaplan et al. 2006;Vejvoda et al. 2008). Change of substrate concentration did not produce any effect on enatioselectivity of Alcaligenes sp. MTCC 10675 arylacetonitrilase (Yamamoto et al. 1992). Heavy metals, such as Ag+, Hg2+, and Pb2+, were strong inhibitor of arylacetonitrilase activity as these metal ions have strong affinities for SH groups of cystine present at the active site of arylacetonitrilase of Alcaligenes sp. MTCC 10675. Hg2+, Mg2+, and Cu2+ were strong inhibitor of arylacetonitrilase activity of Alcaligenes sp. ECU0401 (Zhang et al. 2011). Metal ion inhibition study revealed that enzyme contained sulfhydral (−SH), alcohol, or acid groups as part of their active sites, any chemical which can react with these side chain groups of amino acids at the reaction center active site acts as an irreversible inhibitor. Addition of ethanol and methanol at concentration of more than 20 % in the reaction resulted into loss of arylacetonitrilase activity (Banerjee et al. 2006). Polar solvents caused more damage to arylacetonitrilase activity because nonpolar solvents (hydrophobic) do not disrupt the necessary tightly bond water layer around the enzyme required for the activity of hydrolytic enzyme (Yang et al. 2004). A number of bacteria have been used for the production of mandelic acid, but most of them had lower yield in comparison to the bioresource being reported in this communication (Table 5), e.g., P. putida MTCC 5110 (0.39 gg-1 dcw) and A. faecalis ECU0401 (3.8 gg-1 dcw) as already reported (Banerjee et al. 2006; He et al. 2010). Hydroxy acids are very important organic acids, but very few hydroxy acids are produced commercially by using enzymatic processes due to nonavailability of cheap source of enzyme and technology. Alcaligenes sp. MTCC 10675, isolated in this study, has high biocatalytic activity and can hydrolyze mandelonitrile into mandelic acid very efficiently.
References
Banerjee A, Dubey S, Kaul P, Barse B, Piotrowski M, Banerjee UC (2009) Enantioselective nitrilase from Pseudomonas putida: cloning, heterologous expression, and bioreactor studies. Mol Biotechnol 41:35–41
Banerjee A, Kaul P, Banerjee UC (2006) Enhancing the catalytic potential of nitrilase from Pseudomonas putida for stereoselective nitrile hydrolysis. Appl Microbiol Biotechnol 72:77–87
Bhalla TC, Miura M, Wakamoto A, Ohba Y, Furuhashi K (1992) Asymmetric hydrolysis of α-aminonitriles to optically active amino acids by a nitrilase of Rhodococcus rhodochrous PA-34. Appl Microbiol Biotechnol 37:184–190
Bhatia SK, Mehta PK, Bhatia RK, Bhalla TC (2013) An isobutyronitrile induced bienzymatic system of Alcaligenes sp. MTCC 10674 for the production of alpha-hydroxyisobutyric acid. Bioprocess Biosyst Eng 36(5):613–625
Fawcett JK, Scott JE (1969) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Fournand D, Arnaud A, Galzy P (1998) Acyl transfer activity of an amidase from Rhodococcus sp. strain R312: formation of a wide range of hydroxamic acids. J Mol Catal B: Enzyme 4:77–90
Gong JS, Lu ZM, Li H, Shi JS, Zhou ZM, Xu ZH (2012) Nitrilases in nitrile biocatalysis: recent progress and forthcoming research. Microb Cell Fact 11:142
He YC, Xu JH, Su JH, Zhou L (2010) Bioproduction of glycolic acid from glycolonitrile with a new bacterial isolate of Alcaligenes sp. ECU0401. Appl Biochem Biotechnol 160:1428–1440
Jain D, Meena VS, Kaushik S, Kamble A, Chisti Y, Banerjee UC (2012) Production of nitrilase by a recombinant Escherichia coli in a laboratory. Fermenta Technol 1(1)
Kaplan O, Vejvoda V, Charvátová-Pinvejcová A, Martínková L (2006) Hyper induction of nitrilases in filamentous fungi. J Ind Microbiol Biotechnol 33:891–896
Kaul P, Banerjee A, Mayilraj S, Banerjee A (2004) Screening for enantioselective nitrilases: kinetic resolution of racemic mandelonitrile to (R)-(-)-mandelic acid by new bacterial isolates. Tetrahedron Asym 15:207–211
Kobayashi M, Yanaka N, Nagasawa T, Yamada H (1991) Hyper induction of an aliphatic nitrilase by Rhodococcus rhodochrous K22. FEMS Microbiol Lett 77:121–124
Nagasawa T, Kobayashi M, Yamada H (1988) Optimum culture conditions for the production of benzonitrilase by Rhodococcus rhodochrous J1. Arch Microbiol 150:89–94
Nagasawa T, Mauger J, Yamada H (2010) A novel nitrilase, arylacetonitrilase, of Alcaligenes faecalis JM3-purification and characterization. Eur J Biochem 194:765–777
Rustler S, Stolz A (2007) Isolation and characterization of a nitrile hydrolyzing acidotolerant black yeast-Exophiala oligosperma R1. Appl Microbiol Biotechnol 75:899–908
Sosedov O, Baum S, Bürger S, Matzer K, Kiziak C, Stolz A (2010) Construction and application of variants of the Pseudomonas fluorescens EBC191 arylacetonitrilase for increased production of acids or amides. Appl Environ Microbiol 76:3668–3674
Vejvoda V, Kaplan O, Bezouska K, Pompach P, Sulc M, Cantarella M, Benada O, Uhnakova B, Rinagelova A, Lutz-Wahl S, Fischer L, Kren V, Martınkova L (2008) Purification and characterization of a nitrilase from Fusarium solani O1. J Mol Catal B Enzym 50:99–106
Watanabe I, Satoh Y, Enomoto K, Seki S, Sakashita K (1987) Optimal conditions for cultivation of Rhodococcus sp. N-774 and for conversion of acrylonitrile to acrylamide by resting cells. Agric Biol Chem 51:3201–3206
Xue YP, Liua ZQ, Xub M, Wanga YJ, Zhenga YG, Shen YC (2010) Enhanced biotransformation of (R, S)-mandelonitrile to (R)-(-)-mandelic acid with in situ production removal by addition of resin. Biochem Eng J 53:143–149
Yamamoto K, Fujimatsu I, Komatsu K (1992) Purification and characterization of the nitrilase from Alcaligenes faecalis ATCC 8750 responsible for enantioselective hydrolysis of mandelonitrile. J Ferment Bioeng 73:425–430
Yamamoto K, Komatsu K (1991) Purification and characterization of nitrilase responsible for the enantioselective hydrolysis from Acinetobacter sp. AK226. Agric Biol Chem 55:1459–1466
Yamazaki Y, Maeda H (1986) (R)-(-) Mandelic acid and other 2-hydroxycarboxylic acids: screening for the enzyme, and its purification, characterization and use. Agric Biol Chem 50:2621–2631
Yang L, Dordil JS, Gade S (2004) Hydration of enzyme in nonaqueous media is consistent with solvent dependence of its activity. Biophys J 87(2):812–821
Zhang Z, Xu JH, He YC, Ouyang LM, Liu YY (2011) Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. ECU0401 and its potential for (R)-(-)-mandelic acid production. Bioprocess Biosyst Eng 34:39
Zhu D, Mukherjee C, Yang Y, Rios BE, Gallagher DT, Smith NN, Biehl ER, Hua L (2008) A new nitrilase from Bradyrhizobium japonicum USDA 110 gene cloning, biochemical characterization and substrate specificity. J Biotechnol 133:327–333
Acknowledgments
The authors acknowledge the Department of Biotechnology and University Grant Commission, India for financial support in the form of Senior Research Fellowship to Mr. Shashi Kant Bhatia, Praveen Kumar Mehta, and Ravi Kant Bhatia. The computational facility availed at Bioinformatics Centre, Himachal Pradesh University is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bhatia, S.K., Mehta, P.K., Bhatia, R.K. et al. Optimization of arylacetonitrilase production from Alcaligenes sp. MTCC 10675 and its application in mandelic acid synthesis. Appl Microbiol Biotechnol 98, 83–94 (2014). https://doi.org/10.1007/s00253-013-5288-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5288-9