Abstract
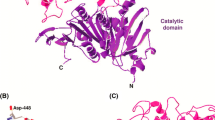
The search for new tannases with novel enzymatic properties suitable for industrial applications has been a continuous effort since the first discovery of the enzyme more than a century ago. A tannase gene (Ss-Tan) from the Gram-positive bacterium Streptomyces sviceus was identified, chemically synthesized, and cloned into a C-terminal His-tagged vector for expression in Escherichia coli. The tannase possesses the active site motif of GXSXG that is conserved for serine hydrolases. The residues that constitute the catalytic triad and galloyl binding site in bacterial tannases are found conserved in Ss-Tan, which include Ser209, Asp452, His484 and Lys370, Glu384, Asp454, respectively. Ss-Tan was overexpressed in E. coli BL21-AI cells with high productivity. Enzymatic assay revealed that the enzyme displays tannase activities to hydrolyze both the ester bonds and depside bonds in hydrolyzable tannins. Kinetic analysis indicated that the enzyme preferentially acts on depside bonds with considerably higher substrate affinity and catalytic efficiency. The enzyme showed maximum activity around pH 8.0 and at 50 °C with the highest melting temperature close to 70 °C. The high depsidase activity and thermostablility of Ss-Tan may make the enzyme suitable for potential industrial applications to achieve complete digestion of hydrolyzable tannins.




Similar content being viewed by others
References
Aguilar CN, Gutierrez-Sanchez G (2001) Review: sources, properties, applications and potential uses of tannin acyl hydrolase. Food Sci Technol Int 7:373–382
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Coronel A, Contreras-Esquivel JC (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76:47–59
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Aslanidis C, de Jong PJ (1990) Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res 18:6069–6074
Bagos PG, Nikolaou EP, Liakopoulos TD, Tsirigos KD (2010) Combined prediction of Tat and Sec signal peptides with hidden Markov models. Bioinformatics 26:2811–2817
Banerjee A, Jana A, Pati BR, Mondal KC, Das Mohapatra PK (2012) Characterization of tannase protein sequences of bacteria and fungi: an in silico study. Protein J 31:306–327
Boadi DK, Neufeld RJ (2001) Encapsulation of tannase for the hydrolysis of tea tannins. Enzyme Microb Technol 28:590–595
Böer E, Breuer FS, Weniger M, Denter S, Piontek M, Kunze G (2011) Large-scale production of tannase using the yeast Arxula adeninivorans. Appl Microbiol Biotechnol 92:105–114
Chater KF, Biró S, Lee KJ, Palmer T, Schrempf H (2010) The complex extracellular biology of Streptomyces. FEMS Microbiol Rev 34:171–198
Curiel JA, Rodríguez H, Acebrón I, Mancheño JM, de las Rivas B, Muñoz R (2009) Production and physicochemical properties of recombinant Lactobacillus plantarum tannase. J Agric Food Chem 57:6224–6230
Farias GM, Gorbea C, Elkins JR, Griffin GJ (1994) Purification, characterization, and substrate relationships of the tannase from Cryphonectria parasitica. Physiol Mol Plant Pathol 44:51–63
Haslam E, Stangroom JE (1966) The esterase and depsidase activities of tannase. Biochem J 99:28–31
Hatamoto O, Watarai T, Kikuchi M, Mizusawa K, Sekine H (1996) Cloning and sequencing of the gene encoding tannase and a structural study of the tannase subunit from Aspergillus oryzae. Gene 175:215–221
He F, Hogan S, Latypov RF, Narhi LO, Razinkov VI (2010) High throughput thermostability screening of monoclonal antibody formulations. J Pharm Sci 99:1707–1720
Hodgson DA (2000) Primary metabolism and its control in Streptomycetes: a most unusual group of bacteria. Adv Microb Physiol 42:47–238
Inoue KH, Hagerman AE (1988) Determination of gallotannin with rhodanine. Anal Biochem 169:363–369
Iwamoto K, Tsuruta H, Nishitaini Y, Osawa R (2008) Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917(T). Syst Appl Microbiol 31:269–277
Jiménez N, Barcenilla JM, de Felipe FL, de las Rivas B, Munoz R (2014a) Characterization of a bacterial tannase from Streptococcus gallolyticus UCN34 suitable for tannin biodegradation. Appl Microbiol Biotechnol 98:6329–6337
Jiménez N, Esteban-Torres M, Mancheño J, de las Rivas B, Munoz R (2014b) Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl Environ Microbiol 80:2991–2997
Khanbabaee K, van Ree T (2001) Tannins: classification and definition. Nat Prod Rep 18:641–649
Knudson L (1913) Tannic acid fermentation. I. J Biol Chem 3:159–184
Kumar S, Tsai CJ, Nussinov R (2000) Factors enhancing protein thermostability. Protein Eng 13:179–191
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:215–260
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lu MJ, Chu SC, Yan L, Chen C (2009) Effect of tannase treatment on protein-tannin aggregation and sensory attributes of green tea infusion. LWT-Food Sci Technol 42:338–342
Mizuguchi K, Sele M, Cubellis MV (2007) Environment specific substitution tables for thermophilic proteins. BMC Bioinformatics 8:S15. doi:10.1186/1471-2105-8-S1-S15
Niehaus JU, Gross GG (1997) A gallotannin degrading esterase from leaves of pedunculate oak. Phytochemistry 45:1555–1560
Nierenstein M (1930) Galls. Nature 125:348–349
Noguchi N, Ohashi T, Shiratori T, Narui K, Hagiwara T, Ko M, Watanabe K, Miyahara T, Taira S, Moriyasu F, Sasatsu M (2007) Association of tannase-producing Staphylococcus lugdunensis with colon cancer and characterization of a novel tannase gene. J Gastroenterol 42:346–351
Ow Y-Y, Stupans I (2003) Gallic acid and gallic acid derivatives: effects on drug metabolizing enzymes. Curr Drug Metab 4:241–248
Ren B, Wu M, Wang Q, Peng X, Wen H, McKinstry WJ, Chen Q (2013) Crystal structure of tannase from Lactobacillus plantarum. J Mol Biol 425:2737–2751
Rodríguez-Durán LV, Valdivia-Urdiales B, Contreras-Esquivel JC, Rodríguez-Herrera R, Aguilar CN (2011) Novel strategies for upstream and downstream processing of tannin acyl hydrolase. Enzyme Res 2011:823619
Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Madden TL, Maglott DR, Miller V, Mizrachi I, Ostell J, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Yaschenko E, Ye J (2009) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 37:5–15
Seabrook SA, Newman J (2013) High-throughput thermal scanning for protein stability: making a good technique more robust. ACS Comb Sci 15:387–392
Sharma KP, John PJ (2011) Purification and characterization of tannase and tannase gene from Enterobacter sp. Process Biochem 46:240–244
Watve MG, Tickoo R, Jog MM, Bhole BD (2001) How many antibiotics are produced by the genus Streptomyces? Arch Microbiol 176:386–390
Widdick DA, Dilks K, Chandra G, Bottrill A, Naldrett M, Pohlschröder M, Palmer T (2006) The twin-arginine translocation pathway is a major route of protein export in Streptomyces coelicolor. Proc Natl Acad Sci U S A 103:17927–17932
Wu M, Peng X, Wen H, Wang Q, Chen Q, McKinstry WJ, Ren B (2013) Expression, purification, crystallization and preliminary X-ray analysis of tannase from Lactobacillus plantarum. Acta Crystallogr Sect F: Struct Biol Cryst Commun 69:456–459
Zhong X, Peng L, Zheng S, Sun Z, Ren Y, Dong M, Xu A (2004) Secretion, purification, and characterization of a recombinant Aspergillus oryzae tannase in Pichia pastoris. Protein Expr Purif 36:165–169
Acknowledgments
This work was supported by the CSIRO Materials Science and Engineering Bioscience Program and CSIRO Transformational Biology Capability Platform for enhancing recombinant protein production. We thank the Structural Genomics Consortium for the provision of the pNIC-CH cloning vector and Dr. Shane Seabrook for performing DSF. MW visit to CSIRO was supported by the China Scholarship Council (CSC).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
BR conceived the project. MW and QW performed the experiments under the supervision of BR and WJM. BR wrote the manuscript.
Rights and permissions
About this article
Cite this article
Wu, M., Wang, Q., McKinstry, W.J. et al. Characterization of a tannin acyl hydrolase from Streptomyces sviceus with substrate preference for digalloyl ester bonds. Appl Microbiol Biotechnol 99, 2663–2672 (2015). https://doi.org/10.1007/s00253-014-6085-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6085-9