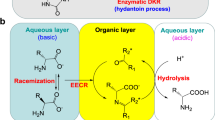
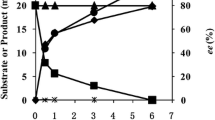
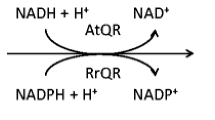
Abstract
Chiral resolutions of racemic mixtures are limited to a theoretical yield of 50 %. This yield can be doubled by integration of a step-wise or continuous racemization of the non-desired enantiomer. Many of the different routes along which the racemization step can be conducted require harsh treatments and are therefore often incompatible with the highly functionalized state of many compounds relevant for the life science industries. Employing enzymatic catalysis for racemization can therefore be highly beneficial. Racemases allow racemization in one reaction step. Most representatives from this group are found in the domain of amino acid or amino acid derivative racemization, with few other examples, notably the racemization of mandelic acid. Corresponding to the importance of enantiospecific conversion of amino acid precursor racemates for the production of enantiopure amino acids, the most important biotechnological use for racemases is the racemization of such precursors. However, alternative uses, in particular for mandelate and amino acid racemases, are emerging. Here, we summarize the natural roles of racemases and their occurrence, the applications, and the biochemistry and engineering of this promising class of biocatalysts.







Similar content being viewed by others
References
Ahmed SA, Esaki N, Tanaka H, Soda K (1983) Racemization of α-Amino-δ-valerolactam catalyzed by α-Amino-ε-caprolactam racemase from Achromobacter obae. Agric Biol Chem 47:1149–1150
Asano Y, Endo K (1988) Amino acid racemase with broad substrate specificity, its properties and use in phenylalanine racemization. Appl Microbiol Biotechnol 29(6):523–527
Asano Y, Hölsch K (2012) Isomerizations enzyme catalysis in organic synthesis. Wiley-VCH Verlag GmbH & Co. KGaA, pp 1607–1684. doi:10.1002/9783527639861.ch39
Asano Y, Yamaguchi S (2005) Discovery of amino acid amides as new substrates for α-amino-epsilon-caprolactam racemase from Achromobacter obae. J Mol Catal B Enzym 36:22–29
Bae HS, Lee SG, Hong SP, Kwak MS, Esaki N, Soda K, Sung MH (1999) Production of aromatic D-amino acids from α-keto acids and ammonia by coupling of four enzyme reactions. J Mol Catal B Enzym 6:241–247
Bae HS, Hong SP, Lee SG, Kwak MS, Esaki N, Sung MH (2002) Application of a thermostable glutamate racemase from Bacillus sp SK-1 for the production of D-phenylalanine in a multi-enzyme system. J Mol Catal B Enzym 17:223–233
Baldea M (2015) From process integration to process intensification. Comput Chem Eng 81:104–114
Bechtold M, Makart S, Heinemann M, Panke S (2006) Integrated operation of continuous chromatography and biotransformations for the generic high yield production of fine chemicals. J Biotechnol 124:146–162
Bechtold M, Makart S, Reiss R, Alder P, Panke S (2007) Model-based characterization of an amino acid racemase from Pseudomanas putida DSM 3263 for application in medium-constrained continuous processes. Biotechnol Bioeng 98:812–824
Bhaumik P, Kursula P, Ratas V, Conzelmann E, Hiltunen JK, Schmitz W, Wierenga RK (2003) Crystallization and preliminary X-ray diffraction studies of an α-methylacyl-CoA racemase from Mycobacterium tuberculosis. Acta Crystallogr Sect D: Biol Crystallogr 59:353–355
Bodanszky M, Perlman D (1969) Peptide Antibiotics. Science 163:352–358
Bosshart A, Panke S, Bechtold M (2013) Systematic optimization of interface interactions increases the thermostability of a multimeric enzyme. Angew Chem Int Ed 52:9673–9676
Bourque JR, Bearne SL (2007) Mutational analysis of the active site flap (20s loop) of mandelate racemase. Biochemistry 47:566–578
Buschiazzo A, Goytia M, Schaeffer F, Degrave W, Shepard W, Grégoire C, Chamond N, Cosson A, Berneman A, Coatnoan N, Alzari PM, Minoprio P (2006) Crystal structure, catalytic mechanism, and mitogenic properties of Trypanosoma cruzi proline racemase. Proc Natl Acad Sci U S A 103:1705–1710
Caboche S, Pupin M, Leclere V, Fontaine A, Jacques P, Kucherov G (2008) NORINE: a database of nonribosomal peptides. Nucleic Acids Res 36:D326–D331
Caboche S, Leclere V, Pupin M, Kucherov G, Jacques P (2010) Diversity of monomers in nonribosomal peptides: towards the prediction of origin and biological activity. J Bacteriol 192:5143–5150
Chakiath C, Lyons MJ, Kozak RE, Laufer CS (2009) Thermal stabilization of Erwinia chrysanthemi pectin methylesterase A for application in a sugar beet pulp biorefinery. Appl Environ Microbiol 75:7343–7349
Chen H-P, Lin C-F, Lee Y-J, Tsay S-S, Wu S-H (2000) Purification and properties of ornithine racemase from Clostridium sticklandii. J Bacteriol 182:2052–2054
Conti E, Stachelhaus T, Marahiel MA, Brick P (1997) Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin. S EMBO J 16:4174–4183
Conti P, Tamborini L, Pinto A, Blondel A, Minoprio P, Mozzarelli A, De Micheli C (2011) Drug discovery targeting amino acid racemases. Chem Rev 111:6919–6946
Counago R, Davlieva M, Strych U, Hill R, Krause K (2009) Biochemical and structural characterization of alanine racemase from Bacillus anthracis (Ames). BMC Struct Biol 9:53
Courvalin P (2006) Vancomycin resistance in gram-positive cocci. Clin Infect Dis 42:S25–S34
Crosby J (1991) Synthesis of optically-active compounds—a large-scale perspective. Tetrahedron 47:4789–4846
Ebbers EJ, Ariaans GJA, Houbiers JPM, Bruggink A, Zwanenburg B (1997) Controlled racemization of optically active organic compounds: prospects for asymmetric transformation. Tetrahedron 53:9417–9476
Eijsink VGH, Bjork A, Gaseidnes S, Sirevag R, Synstad B, van den Burg B, Vriend G (2004) Rational engineering of enzyme stability. J Biotechnol 113:105–120
Eijsink VGH, Gaseidnes S, Borchert TV, van den Burg B (2005) Directed evolution of enzyme stability. Biomol Eng 22:21–30
Espaillat A, Carrasco-López C, Bernardo-García N, Pietrosemoli N, Otero LH, Álvarez L, de Pedro MA, Pazos F, Davis BM, Waldor MK, Hermoso JA, Cava F (2014) Structural basis for the broad specificity of a new family of amino-acid racemases. Acta Crystallogr D Biol Crystallogr 70:79–90
Felfer U, Goriup M, Koegl MF, Wagner U, Larissegger-Schnell B, Faber K, Kroutil W (2005) The substrate spectrum of mandelate racemase: minimum structural requirements for substrates and substrate model. Adv Synth Catal 347:951–961
Ferrer M, Golyshina O, Beloqui A, Golyshin PN (2007) Mining enzymes from extreme environments. Curr Opin Microbiol 10:207–214
Fisher LM, Albery WJ, Knowles JR (1986) Energetics of proline racemase: racemization of unlabeled proline in the unsaturated, saturated, and oversaturated regimes. Biochemistry 25:2529–2537
Fuereder M, Majeed IN, Panke S, Bechtold M (2014) Model-based identification of optimal operating conditions for amino acid simulated moving bed enantioseparation using a macrocyclic glycopeptide stationary phase. J Chromatog A 1346:34–42
Fuereder M, Femmer C, Storti G, Panke S, Bechtold M (2016) Integration of simulated moving bed chromatography and enzymatic racemization for the production of single enantiomers. Chem Eng Sci. doi:10.1016/j.ces.2016.05.033, Available online 16 June 2016
Fujii N (2002) D-amino acids in living higher organisms. Orig Life Evol Biosph 32:103–127
Fukumura T (1973) Process for preparing L-lysine. Google Patents
Fukumura T (1977) Enzymatic conversion of DL-α-Amino-ε-caprolactam into L-Lysine. 4. Partial-purification and some properties of α-Amino-ε-caprolactam-racemizing enzyme from Achromobacter obae. Agric Biol Chem 41:1509–1510
Gao XZ, Ma QY, Zhu HL (2015) Distribution, industrial applications, and enzymatic synthesis of D-amino acids. Appl Microbiol Biotechnol 99:3341–3349
Gaseidnes S, Synstad B, Jia XH, Kjellesvik H, Vriend G, Eijsink VGH (2003) Stabilization of a chitinase from Serratia marcescens by Gly -> Ala and Xxx -> Pro mutations. Protein Eng 16:841–846
Goffin P, Deghorain M, Mainardi J-L, Tytgat I, Champomier-Verges M-C, Kleerebezem M, Hols P (2005) Lactate racemization as a rescue pathway for supplying D-lactate to the cell wall biosynthesis machinery in Lactobacillus plantarum. J Bacteriol 187:6750–6761
Goto M (2010) Crystal structure of serine racemase that produces neurotransmitter D-serine for stimulation of the NMDA receptor. J Crystallogr 52:120–124, Society of Japan
Goto M, Yamauchi T, Kamiya N, Miyahara I, Yoshimura T, Mihara H, Kurihara T, Hirotsu K, Esaki N (2009) Crystal structure of a homolog of mammalian serine racemase from Schizosaccharomyces pombe. J Biol Chem 284:25944–25952
Gribenko AV, Patel MM, Liu J, McCallum SA, Wang CY, Makhatadze GI (2009) Rational stabilization of enzymes by computational redesign of surface charge-charge interactions. Proc Natl Acad Sci U S A 106:2601–2606
He M (2006) Pipecolic acid in microbes: biosynthetic routes and enzymes. J Ind Microbiol Biotechnol 33:401–407
Huerta FF, Minidis ABE, Backvall J-E (2001) Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem Soc Rev 30:321–331
Hwang KY, Cho C-S, Kim SS, Sung H-C, Yu YG, Cho Y (1999) Structure and mechanism of glutamate racemase from Aquifex pyrophilus. Nat Struct Mol Biol 6:422–426
Ishikawa T, Watabe K, Mukohara Y, Nakamura H (1997) Mechanism of stereospecific conversion of DL-5-substituted hydantoins to the corresponding L-amino acids by Pseudomonas sp. strain NS671. Biosci Biotechnol Biochem 61:185–187
Ishiwata KI, Fukuhara N, Shimada M, Makiguchi N, Soda K (1990) Ezymatic production of L-Tryptophan from DL-Serine and indole by a coupled reaction of tryptophan synthase and amino acid racemase. Biotechnol Appl Biochem 12:141–149
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Kaspereit M, Swernath S, Kienle A (2012) Evaluation of competing process concepts for the production of pure enantiomers. Org Process Res Dev 16:353–363
Kim PM, Duan X, Huang AS, Liu CY, Ming G-l, Song H, Snyder SH (2010) Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci U S A 107:3175–3179
Kino K, Sato M, Yoneyama M, Kirimura K (2007) Synthesis of DL-tryptophan by modified broad specificity amino acid racemase from Pseudomonas putida IFO 12996. Appl Microbiol Biotechnol 73:1299–1305
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) D-Amino acids trigger biofilm disassembly. Science 328:627–629
Korkegian A, Black ME, Baker D, Stoddard BL (2005) Computational thermostabilization of an enzyme. Science 308:857–860
Lehmann M, Pasamontes L, Lassen SF, Wyss M (2000) The consensus concept for thermostability engineering of proteins. Biochim Biophys Acta 1543:408–415
Lundqvist T, Fisher SL, Kern G, Folmer RH, Xue Y, Newton DT, Keating TA, Alm RA, de Jonge BL (2007) Exploitation of structural and regulatory diversity in glutamate racemases. Nature 447:817–822
Martinez-Rodriguez S, Javier Las Heras-Vazquez F, María Clemente-Jimenez J, Mingorance-Cazorla L, Rodriguez-Vico F (2002) Complete conversion of D, L-5-monosubstituted hydantoins with a low velocity of chemical racemization into D-amino acids using whole cells of recombinant Escherichia coli. Biotechnol Prog 18:1201–1206
Martinez-Rodriguez S, Gonzalez-Ramirez LA, Clemente-Jimenez JM, Rodriguez-Vico F, Las Heras-Vazquez FJ, Gavira JA, Garcia-Ruiz JM (2008) Crystallization and preliminary crystallographic studies of an active-site mutant hydantoin racemase from Sinorhizobium meliloti CECT4114 Acta Crystallogr. F-Struct. Biol Cryst Commun 64:50–53
Matsui D, Oikawa T, Arakawa N, Osumi S, Lausberg F, Stäbler N, Freudl R, Eggeling L (2009) A periplasmic, pyridoxal-5′-phosphate-dependent amino acid racemase in Pseudomonas taetrolens. Appl Microbiol Biotechnol 83:1045–1054
May O, Verseck S, Bommarius A, Drauz K (2002) Development of dynamic kinetic resolution processes for biocatalytic production of natural and nonnatural L-amino acids. Org Process Res Dev 6:452–457
May M, Mehboob S, Mulhearn DC, Wang Z, Yu H, Thatcher GR, Santarsiero BD, Johnson ME, Mesecar AD (2007) Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications for inhibitor design. J Mol Biol 371:1219–1237
Muramatsu H, Mihara H, Yasuda M, Ueda M, Kurihara T, Esaki N (2006) Enzymatic synthesis of L-pipecolic acid by Δ1-piperideine-2-carboxylate reductase from Pseudomonas putida. Biosci Biotechnol Biochem 70:2296–2298
Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH (2007) Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-Serine formation. Proc Natl Acad Sci U S A 104:2950–2955
Nagata Y, Horiike K, Maeda T (1994) Distribution of free D-serine in vertebrate brains. Brain Res 634:291–295
Nara TY, Togashi H, Sekikawa C, Inoh K, Hisamatsu K, Sakaguchi K, Mizukami F, Tsunoda T (2010) Functional immobilization of racemase by adsorption on folded-sheet mesoporous silica. J Mol Catal B Enzym 64:107–112
Nestl BM, Glueck SM, Hall M, Kroutil W, Stuermer R, Hauer B, Faber K (2006) Biocatalytic racemization of (hetero)aryl-aliphatic α-hydroxycarboxylic acids by Lactobacillus spp. Proceeds via an Oxidation–Reduction Sequence. Eur J Org Chem 2006:4573–4577
Oikawa T, Watanabe M, Makiura H, Kusakabe H, Yamade K, Soda K (1999) Production of D-glutamate from L-glutamate with glutamate racemase and L-glutamate oxidase. Biosci Biotechnol Biochem 63:2168–2173
Okazaki S, Suzuki A, Mizushima T, Kawano T, Komeda H, Asano Y, Yamane T (2009) The novel structure of a pyridoxal 5′-phosphate-dependent fold-type I racemase, α-amino-ε-caprolactam racemase from Achromobacter obae. Biochemistry 48:941–950
Okubo Y, Yokoigawa K, Esaki N, Soda K, Misono H (2000) High catalytic activity of alanine racemase from psychrophilic Bacillus psychrosaccharolyticus at high temperatures in the presence of pyridoxal 5′-phosphate FEMS. Microbiol Lett 192:169–173
Okumura I, Yamamoto T (1978) Enzymic racemization of allantoin. J Biochem 84:891–895
Pellissier H (2003) Dynamic kinetic resolution. Tetrahedron 59:8291–8327
Pellissier H (2008) Recent developments in dynamic kinetic resolution. Tetrahedron 64:1563–1601
Pope SD, Chen L-L, Stewart V (2009) Purine utilization by Klebsiella oxytoca M5al: genes for ring-oxidizing and -opening enzymes. J Bacteriol 191:1006–1017
Porter JL, Rusli RA, Ollis DL (2016) Directed evolution of enzymes for industrial biocatalysis. Chembiochem 17:197–203
Pozo-Dengra J, Isabel Martinez-Gomez A, Martinez-Rodriguez S, Maria Clemente-Jimenez J, Rodriguez-Vico F, Las Heras-Vazquez FJ (2009) Racemization study on different N-acetylamino acids by a recombinant N-succinylamino acid racemase from Geobacillus kaustophilus CECT4264. Process Biochem 44:835–841
Radkov AD, Moe LA (2014) Bacterial synthesis of D-amino acids. Appl Microbiol Biotechnol 98:5363–5374
Reetz MT, DCarballeira J, Vogel A (2006) Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew Chem Int Ed 45:7745–7751
Rubinstein A, Major DT (2009) Catalyzing racemizations in the absence of a cofactor: the reaction mechanism in proline racemase. J Am Chem Soc 131:8513–8521
Schell MJ, Cooper OB, Snyder SH (1997) D-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci U S A 94:2013–2018
Schmitz W, Fingerhut R, Conzelmann E (1994) Purification and properties of an α-methylacyl-CoA racemase from rat liver. Eur J Biochem 222:313–323
Schmitz W, Albers C, Fingerhut R, Conzelmann E (1995) Purification and characterization of an α-methylacyl-CoA racemase from human liver. Eur J Biochem 231:815–822
Schnell B, Faber K, Kroutil W (2003) Enzymatic racemisation and its application to synthetic biotransformations. Adv Synth Catal 345:653–666
Sheldon RA (1996) Chirotechnology: designing economic chiral syntheses. J Chem Technol Biotechnol 67:1–14
Smith MA, Mack V, Ebneth A, Moraes I, Felicetti B, Wood M, Schonfeld D, Mather O, Cesura A, Barker J (2010) The structure of mammalian serine racemase. J Biol Chem 285:12873–12881
Soda K, Tanaka H, Tanizawa K (1988) Thermostable alanine racemase and its application to D-Amino acid synthesis. In: Blanch HW, Klibanov AM editors. pp 375-382. Ann NY Acad Sci
Sonke T, Kaptein B (2012) Hydrolysis of Amides Enzyme Catalysis in Organic Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA, pp 561–650. doi:10.1002/9783527639861.ch15
Stecher H, Felfer U, Faber K (1997) Large-scale production of Mandelate racemase by Pseudomonas putida ATCC 12633: optimization of enzyme induction and development of a stable crude enzyme preparation. J Biotechnol 56:33–40
Suzuki S, Onishi N, Yokozeki K (2005) Purification and characterization of hydantoin racemase from Microbacterium liquefaciens AJ 3912. Biosci Biotechnol Biochem 69:530–536
Tani Y, Miyake R, Yukami R, Dekishima Y, China H, Saito S, Kawabata H, Mihara H (2015) Functional expression of L-lysine α-oxidase from Scomber japonicus in Escherichia coli for one-pot synthesis of L-pipecolic acid from DL-lysine. Appl Microbiol Biotechnol 99:5045–5054
Tokuyama S, Hatano K, Takahashi T (1994) Discovery of a novel enzyme, N-acylamino acid racemase in an actinomycete: screening, isolation, and identification. Biosci Biotechnol Biochem 58:24–27
Toth K, Richard JP (2007) Covalent catalysis by pyridoxal: evaluation of the effect of the cofactor on the carbon acidity of glycine. J Am Chem Soc 129:3013–3021
Vranova V, Zahradnickova H, Janous D, Skene KR, Matharu AS, Rejsek K, Formanek P (2012) The significance of D-amino acids in soil, fate and utilization by microbes and plants: review and identification of knowledge gaps. Plant Soil 354:21–39
Wagner N, Fuereder M, Bosshart A, Panke S, Bechtold M (2011) Practical aspects of integrated operation of biotransformation and SMB separation for fine chemical synthesis. Org Process Res Dev 16:323–330
Wagner N, Bosshart A, Failmezger J, Bechtold M, Panke S (2015a) A separation-integrated cascade reaction to overcome thermodynamic limitations in rare-sugar synthesis. Angew Chem Int Ed 127:4256–4260
Wagner N, Bosshart A, Wahler S, Failmezger J, Panke S, Bechtold M (2015b) Model-based cost optimization of a reaction-separation integrated process for the enzymatic production of the rare sugar D-psicose at elevated temperatures. Chem Eng Sci 137:423–435
Wiese A, Pietzsch M, Syldatk C, Mattes R, Altenbuchner J (2000) Hydantoin racemase from Arthrobacter aurescens DSM 3747: heterologous expression, purification and characterization. J Biotechnol 80:217–230
Willies SC, White JL, Turner NJ (2012) Development of a high-throughput screening method for racemase activity and its application to the identification of alanine racemase variants with activity towards L-arginine. Tetrahedron 68:7564–7567
Wu D, Hu T, Zhang L, Chen J, Du J, Ding J, Jiang H, Shen X (2008) Residues Asp164 and Glu165 at the substrate entryway function potently in substrate orientation of alanine racemase from E. coli: enzymatic characterization with crystal structure analysis. Protein Sci 17:1066–1076
Würges K, Petruševska-Seebach K, Elsner MP, Lütz S (2009) Enzyme-assisted physicochemical enantioseparation processes—part III: overcoming yield limitations by dynamic kinetic resolution of asparagine via preferential crystallization and enzymatic racemization. Biotechnol Bioeng 104:1235–1239
Würges K, Mackfeld U, Pohl M, Lütz S, Wilhelm S, Wiechert W, Kubitzki T (2011) An efficient route to both enantiomers of allo-threonine by simultaneous amino acid racemase-catalyzed isomerization of threonine and crystallization. Adv Synth Catal 353(13):2431–2438
Yamada H, Shimizu S, Shimada H, Tani Y, Takahashi S, Ohashi T (1980) Production of D-Phenylglycine related amino acids by immobilized microbial cells. Biochimie 62:395–399
Yamaguchi S, Komeda H, Asano Y (2007) New enzymatic method of chiral amino acid synthesis by dynamic kinetic resolution of amino acid amides: use of stereoselective amino acid amidases in the presence of α-amino-epsilon-caprolactam racemase. Appl Environ Microb 73:5370–5373
Yamauchi T, Choi SY, Okada H, Yohda M, Kumagai H, Esaki N, Soda K (1992) Properties of aspartate racemase, a pyridoxal 5′-phosphate-independent amino acid racemase. J Biol Chem 267:18361–18364
Acknowledgements
CF has been financially supported by the EU-funded project INTENANT (contract number 214129).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Femmer, C., Bechtold, M., Roberts, T.M. et al. Exploiting racemases. Appl Microbiol Biotechnol 100, 7423–7436 (2016). https://doi.org/10.1007/s00253-016-7729-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7729-8