Abstract
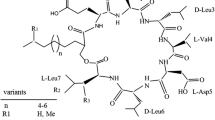
The name surfactin refers to a bacterial cyclic lipopeptide, primarily renowned for its exceptional surfactant power since it lowers the surface tension of water from 72 mN m−1 to 27 mN m−1 at a concentration as low as 20 μM. Although surfactin was discovered about 30 years ago, there has been a revival of interest in this compound over the past decade, triggered by an increasing demand for effective biosurfactants for difficult contemporary ecological problems. This simple molecule also looks very promising as an antitumoral, antiviral and anti-Mycoplasma agent. Structural characteristics show the presence of a heptapeptide with an LLDLLDL chiral sequence linked, via a lactone bond, to a β-hydroxy fatty acid with 13–15 C atoms. In solution, the molecule exhibits a characteristic “horse saddle” conformation that accounts for its large spectrum of biological activity, making it very attractive for both industrial applications and academic studies. Surfactin biosynthesis is catalysed non-ribosomally by the action of a large multienzyme complex consisting of four modular building blocks, called the surfactin synthetase. The biosynthetic activity involves the multicarrier thiotemplate mechanism and the enzyme is organized in structural domains that place it in the family of peptide synthetases, a class of enzymes involved in peptidic secondary-metabolite synthesis. The srfA operon, the sfp gene encoding a 4′-phosphopantetheinyltransferase and the comA regulatory gene work together for surfactin biosynthesis, while the gene encoding the acyltransferase remains to be isolated. Concerning surfactin production, there is no indication whether the genetic regulation, involving a quorum-sensing mechanism, overrides other regulation factors promoted by the fermentation conditions. Knowledge of the modular arrangement of the peptide synthetases is of the utmost relevance to combinatorial biosynthetic approaches and has been successfully used at the gene level to modify the surfactin template. Biosynthetic and genetic rationales have been described for building variants. A fine study of the structure/function relationships associated with the three-dimensional structure has led to the recognition of the specific residues required for activity. These studies will assist researchers in the selection of molecules with improved and/or refined properties useful in oil and biomedical industries.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 9 October 1998 / Received revision: 29 January 1999 / Accepted: 31 January 1999
Rights and permissions
About this article
Cite this article
Peypoux, F., Bonmatin, J. & Wallach, J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51, 553–563 (1999). https://doi.org/10.1007/s002530051432
Issue Date:
DOI: https://doi.org/10.1007/s002530051432