Abstract
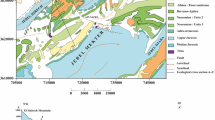
Hydrochemical investigations were carried out in Bahar area, Hamadan, western Iran, to assess the chemical composition of groundwater. The area falls in a semi-arid type of climate. In this area, groundwater has been exploited over the past century mainly for irrigation and water supply. A total of 135 representative groundwater samples were collected from different wells to monitor the water chemistry of various ions. Chemical analysis of the groundwater shows that the mean concentration of the cations is of the order Ca2+>Mg 2+>Na+>K+, while that for anions is SO 2−4 >HCO −3 >Cl−>NO −3 . Statistical analyses indicate positive correlation between the following pairs of parameters Cl− and Mg 2+ (r=0.71), Cl− and Na+ (r=0.76), HCO −3 and Na+ (r=0.56), SO 2−4 and Mg2+ (r=0.76), SO 2−4 and Na+ (r=0.69). Water presents a large spatial variability of the chemical facies (Ca-HCO3, Ca-SO4, Mg-HCO3, Mg-SO4, Na-HCO3) which is in relation to their interaction with the geological formations of the basin (carbonates, dolomite and various silicates) and evaporation. The hydrochemical types Ca-HCO3 and Ca-SO4 dominate the largest part of the studied area. The dissolution of halite, calcite, dolomite, and gypsum explains part of the contained Na +, Ca2+, Mg2+, Cl−, SO 2−4 and HCO −3 , but other processes, such as cation exchange and weathering of aluminosilicates also contribute to the water composition.









Similar content being viewed by others
References
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland, Australia. J Hydrol 130:299–338
Drever JI (1988) The geochemistry of natural waters, 2nd edn. Prentice Hall, Englewood Cliffs
Edmunds WM, Cook JM, Darling WG, Kinniburgh DG, Miles DL, Bath AH, Morgan-Jones M, Andrews JN (1987) Baseline geochemical conditions in the Chalk aquifer, Berkshire, UK: a basis for groundwater quality management. Appl Geochem 2:251–274
Garcia MG, del v Hidalgo M, Blessa MA (2001) Geochemistry of groundwater in the alluvial plain of Tucuman province, Argentina. Hydrogeol J 9:597–610
Hidalgo MC, Cruz-Sanjulian J (2001) Groundwater composition, hydrochemical evolution and mass transfer in a regional detrital aquifer (Baza basin, southern Spain). Appl Geochem 16:745–758
Hidalgo MC, Cruz-Sanjulian J, Sanroma A (1995) Evolucion geoquimica de las aguas subterraneas en una cuenca sedimentaria semiarida (acuifero de Baza-Caniles, Granada, Espana). Tierra y Tecnol 20:39–48
Jalali M (1999) The potassium quantity intensity relationships and the effect of applied potassium on exchangeable and solution potassium in some Hamadan soils. Research report, Bu-Ali-Sina University
Magaritz M, Nadler A, Koyumdjisky H, Dan N (1981) The use of Na/Cl ratio to trace solute sources in a semiarid zone. Water Resour Res 17:602–608
Mathess G (1982) The properties of groundwater. Wiley, New York
McLean W, Jankowski J, Lavitt N (2000) Groundwater quality and sustainability in an alluvial aquifer, Australia. In: Sililo O et al. (ed) Groundwater, past achievements and future challenges. A Balkema, Rotterdam, pp 567–573
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Parkhurst DL, Appelo CAJ (1999) Users guide to PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water Resources Investigations Report 99-4259
Rowell DL (1994) Soil science: methods and applications. Longman Scientific and Technical
Sabziparvar AA (2003) The analysis of aridity and meteorological drought indices in west of Iran. Research report. Bu-Ali Sina University
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern cape, South Africa. J Hydrol 139:27–48
Acknowledgements
The author wishes to thank Dr. D.L. Rowell (Reading University, England) and an anonymous reviewer, whose critical comments greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalali, M. Major ion chemistry of groundwaters in the Bahar area, Hamadan, western Iran. Environ Geol 47, 763–772 (2005). https://doi.org/10.1007/s00254-004-1200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-004-1200-3