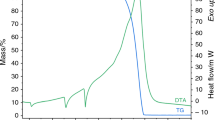
Abstract
Crystallisation of sodium sulfate solutions by evaporation under controlled climatic conditions has revealed the existence of crystalline hydrated sodium sulfate salts not previously reported. The sodium sulfate phase crystallising and the concentration of the solution at the point of crystallisation depends on the climatic conditions (temperature and evaporation rate). During the rehydration of the anhydrous sodium sulfate phase, thenardite, another previously unreported phase was formed prior to the nucleation of the stable phase, mirabilite Na2SO4 · 10H2O. The addition of organic inhibitors changes both the crystallisation and the rehydration behavior in this system.






Similar content being viewed by others
References
Benavente D, Garcia del Cura MA, Garcia-Guinea J, Sanchez-Moral S, Ordonez S (2004) Role of pore structure in salt crystallisation in unsaturated porous stone. J Cryst Growth 260:532–544
Charola AE, Weber J (1992) The hydration and deterioration mechanism of sodium sulfate. In: Delagado Rodrigues J, Henriques F, Telmo Jeremias F (eds) 7th International congress on deterioration and conservation of stone. Laboratório Nacional de Engenharia Civil, Lisbon, pp 581–590
Chaterji S (2000) A discussion of the paper “Crystallisation in pores” by G.W. Scherer. Cem Concr Res 30:669–671
Correns CW (1949) Growth and dissolution of crystals under linear pressure. Discuss Faraday Soc 5:267–271
Flatt RJ (2002) Salt damage in porous materials: how high supersaturations are generated. J Cryst Growth 242:435–454
Füredi-Milhofer H, Babic-Ivancic V, Brecevic L, Filipovic-Vincekovic N, Kralj D, Komunjer L, Markovic M, Skrtic D (1990) Factors influencing nucleation from solutions of supersaturated to different crystal hydrates. Colloids Surf 48:219–230
Goudie AS, Viles H (1997) Salt weathering hazards. Wiley, Chichester, 241 p
Hawthorne FC, Ferguson RB (1975) Anhydrous sulfates. I: Refinement of the crystal structure of celestite with an appendix on the structure of thenardite. Can Mineral 13:181–187
Knacke O, von Erdberg R (1975) The crystallisation pressure of sodium sulfate decahydrate. Ber Bunsen Ges 79:653–657
Levy HA, Lisensky GC (1978) Crystal structures of sodium sulfate decahydrate (Glauber’s salt) and sodium tetraborate decahydrate (Borax) redetermination by neutron diffraction. Acta Cryst B 34:3502–3510
Mehrotra BN, Hahn T, Eysel W, Röpke H, Illguth A (1978) Crystal chemistry of compounds with thenardite structure. Neues Jahrb Mineral Monatsh 408–421
Mehrotra B (1987) Acta Cryst., A43: 119 JCPDS 2002 Card 40–0727
Meller N, Hall C, Jupe AC, Colston SL, Jacques SDM, Barnes P, Phipps J (2004) The paste hydration of brownmillerite with and without gypsum: a time resolved synchrotron diffraction study at 30, 70, 100 and 150 °C. J Mater Chem 14:428–435
Nord AG (1973) Refinement of the crystal structure of thenardite, Na2SO4 (V). Acta Chem Scand 27:814–822
Nyvlt J (1983) Induction period of nucleation and metastable zone width. Collect Czech Chem Commun 48:1977–1983
Putnis A (2002) Mineral replacement reactions: from macroscopic observations to microscopic mechanisms. Mineral Mag 66:689–708
Putnis A, Prieto M, Fernandez-Diaz L (1995) Fluid supersaturation and crystallisation in porous media. Geol Mag 132(1):1–13
Rodriguez-Navarro C, Doehne E (1999) Salt weathering: influence of evaporation rate, supersaturation and crystallisation pattern. Earth Surf Process Landf 24:191–209
Rodriguez-Navarro C, Doehne E, Sebastian E (2000) How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem Concr Res 30:1527–1534
Ruben HW, Templeton DH, Rosenstein RD, Olovsson I (1961) Crystal structure and entropy of sodium sulfate decahydrate. J Am Chem Soc 83:820–824
Scherer GW (2000) Reply to the discussion by S. Chatterji of the paper “Crystallisation in pores”. Cem Concr Res 30:673–675
Scherer GW (2004) Stress from crystallization of salt. Cem Concr Res 34:1613–1624
Steiger M, Beyer R, Dorn J, Zeunert A (2000) Data compilation and experimental determinations. In: Price C (ed) An expert chemical model for determining the environmental conditions needed to prevent salt damage in porous materials (contract no. ENV4-CT95–0135). EU research report no. 11
Steiger M (2005a) Crystal growth in porous materials—I: The crystallization pressure of large crystals. J Cryst Growth 282:455–469
Steiger M (2005b) Crystal growth in porous materials—II: Influence of crystal size on the crystallization pressure. J Cryst Growth 282:470–481
Werner P-E, Eriksson L, Westdahl M (1985) TREOR, a semi-exhaistive trial-and-error powder indexing program for all symmetries. J Appl Cryst 18:367–370
Wetmore FEW, LeRoy DJ (1951) Principles of phase equilibria. McGraw-Hill Book Company, New York, p 200
Acknowledgment
This work is funded through an EU STREP project (Contract SSP1-CT-2003–501571-SALTCONTROL). We thank J. Schumacher and R. Thewes for the construction of the climate chamber for the crystallisation experiments, A. Breit and V. Rapelius for technical help and P. Schmid-Beurmann for running the TREOR program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genkinger, S., Putnis, A. Crystallisation of sodium sulfate: supersaturation and metastable phases. Environ Geol 52, 329–337 (2007). https://doi.org/10.1007/s00254-006-0565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0565-x