Abstract
Objective
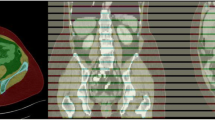
To develop a deep convolutional neural network (CNN) to automatically segment an axial CT image of the pelvis for body composition measures. We hypothesized that a deep CNN approach would achieve high accuracy when compared to manual segmentations as the reference standard.
Materials and methods
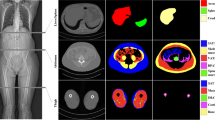
We manually segmented 200 axial CT images at the supra-acetabular level in 200 subjects, labeling background, subcutaneous adipose tissue (SAT), muscle, inter-muscular adipose tissue (IMAT), bone, and miscellaneous intra-pelvic content. The dataset was randomly divided into training (180/200) and test (20/200) datasets. Data augmentation was utilized to enlarge the training dataset and all images underwent preprocessing with histogram equalization. Our model was trained for 50 epochs using the U-Net architecture with batch size of 8, learning rate of 0.0001, Adadelta optimizer and a dropout of 0.20. The Dice (F1) score was used to assess similarity between the manual segmentations and the CNN predicted segmentations.
Results
The CNN model with data augmentation of N = 3000 achieved accurate segmentation of body composition for all classes. The Dice scores were as follows: background (1.00), miscellaneous intra-pelvic content (0.98), SAT (0.97), muscle (0.95), IMAT (0.91), and bone (0.92). Mean time to automatically segment one CT image was 0.07 s (GPU) and 2.51 s (CPU).
Conclusions
Our CNN-based model enables accurate automated segmentation of multiple tissues on pelvic CT images, with promising implications for body composition studies.








Similar content being viewed by others
References
Grimaldi A, Richardson C, Stanton W, Durbridge G, Donnelly W, Hides J. The association between degenerative hip joint pathology and size of the gluteus medius, gluteus minimus and piriformis muscles. Man Ther. 2009;14:605–10.
ten Dam L, van der Kooi AJ, Rövekamp F, Linssen WHJP, de Visser M. Comparing clinical data and muscle imaging of DYSF and ANO5-related muscular dystrophies. Neuromuscul Disord. 2014;24:1097–102.
Woodley SJ, Nicholson HD, Livingstone V, Doyle TC, Meikle GR, Macintosh JE, et al. Lateral hip pain: findings from magnetic resonance imaging and clinical examination. J Orthop Sports Phys Ther. 2008;38:313–28.
Pfirrmann CWA, Notzli HP, Dora C, Hodler J, Zanetti M. Abductor tendons and muscles assessed at MR imaging after total hip arthroplasty in asymptomatic and symptomatic patients. Radiology. 2005;235:969–76.
Ikezoe T, Mori N, Nakamura M, Ichihashi N. Atrophy of the lower limbs in elderly women: is it related to walking ability? Eur J Appl Physiol. 2011;111:989–95.
Kiyoshige Y, Watanabe E. Fatty degeneration of gluteus minimus muscle as a predictor of falls. Arch Gerontol Geriatr. 2015;60:59–61.
Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14:362–6.
Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33.
Oliveira A, Vaz C. The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol. 2015;34:1673–80.
Chang C-D, Wu JS, Mhuircheartaigh JN, Hochman MG, Rodriguez EK, Appleton PT, et al. Effect of sarcopenia on clinical and surgical outcome in elderly patients with proximal femur fractures. Skelet Radiol. 2018;47:771–7.
Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9:1200–8.
Yoo T, Lo WD, Evans DC. Computed tomography measured psoas density predicts outcomes in trauma. Surgery. 2017;162:377–84.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96.
Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. 2019;290:669–79.
Wang Y, Qiu Y, Thai T, Moore K, Liu H, Zheng B. A two-step convolutional neural network based computer-aided detection scheme for automatically segmenting adipose tissue volume depicting on CT images. Comput Methods Prog Biomed. 2017;144:97–104.
Lee H, Troschel FM, Tajmir S, Fuchs G, Mario J, Fintelmann FJ, et al. Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging. 2017;30:487–98.
Yang YX, Chong MS, Tay L, Yew S, Yeo A, Tan CH. Automated assessment of thigh composition using machine learning for Dixon magnetic resonance images. Magma (New York, NY). 2016;29:723–31.
Momose T, Inaba Y, Choe H, Kobayashi N, Tezuka T, Saito T. CT-based analysis of muscle volume and degeneration of gluteus medius in patients with unilateral hip osteoarthritis. BMC Musculoskelet Disord. 2017;18:457.
Uemura K, Takao M, Sakai T, Nishii T, Sugano N. Volume increases of the gluteus maximus, gluteus medius, and thigh muscles after hip arthroplasty. J Arthroplast. 2016;31:906–912.e1.
Rutten IJG, van Dijk DPJ, Kruitwagen RFPM, Beets-Tan RGH, Olde Damink SWM, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–66.
Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells W, Frangi A, editors. Medical image computing and computer-assisted intervention: Springer; 2015. p. 234–41.
Abadi M, Barham P, Chen J, Chen Z, Davis A, Dean J, et al. TensorFlow: a system for large-scale machine learning. OSDI. usenix.org. 2016. p. 265–283.
Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302.
Strulov Shachar S, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
Hopkins JJ, Sawyer MB. A review of body composition and pharmacokinetics in oncology. Expert Rev Clin Pharmacol. 2017;10:947–56.
Chung H, Cobzas D, Birdsell L, Lieffers J, Baracos V. Automated segmentation of muscle and adipose tissue on CT images for human body composition analysis. Proc SPIE 7261, medical imaging 2009: visualization, image-guided procedures, and modeling, 72610K. 2009.
Popuri K, Cobzas D, Esfandiari N, Baracos V, Jägersand M. Body composition assessment in axial CT images using FEM-based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging. 2016;35:512–20.
Kim YJ, Lee SH, Kim TY, Park JY, Choi SH, Kim KG. Body fat assessment method using CT images with separation mask algorithm. J Digit Imaging. 2013;26:155–62.
Parikh AM, Coletta AM, Yu ZH, Rauch GM, Cheung JP, Court LE, et al. Development and validation of a rapid and robust method to determine visceral adipose tissue volume using computed tomography images. PLoS One. 2017 Aug 31;12(8):e0183515
Kullberg J, Hedström A, Brandberg J, Strand R, Johansson L, Bergström G, et al. Automated analysis of liver fat, muscle and adipose tissue distribution from CT suitable for large-scale studies. Sci Rep. 2017;7:10425.
Grimby G, Kvist H, Grangård U. Reduction in thigh muscle cross-sectional area and strength in a 4-year follow-up in late polio. Arch Phys Med Rehabil. 1996;77:1044–8.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge M-P, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (Bethesda, Md : 1985). 2004;97:2333–8.
Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8:11369.
Funding
This study was funded in part by the NIH Nutrition and Obesity Research Center at Harvard P30 DK040561.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were carried out in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study. The study was approved by the local Institutional Review Board (IRB) and HIPAA compliant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemke, R., Buckless, C.G., Tsao, A. et al. Deep learning for automated segmentation of pelvic muscles, fat, and bone from CT studies for body composition assessment. Skeletal Radiol 49, 387–395 (2020). https://doi.org/10.1007/s00256-019-03289-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03289-8