Abstract
Purpose
To predict the Fuhrman grade of clear cell renal cell carcinoma (ccRCC) with a machine learning classifier based on single- or three-phase computed tomography (CT) images.
Materials and methods
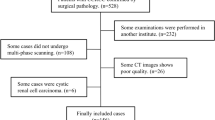
Patients with pathologically proven ccRCC from February 1, 2009 to September 31, 2018 who were not treated were retrospectively collected for machine learning-based analysis. The texture features were extracted and ranked from precontrast phase (PCP), corticomedullary phase (CMP), nephrographic phase (NP) and three-phase CT images, and open-source gradient boosting from the decision tree library of CatBoost was used to establish a machine learning classifier to differentiate low- from high-grade ccRCC. The performances of machine learning classifiers based on features from single- and three-phase CT images were compared with each other.
Results
A total of 231 patients with 232 pathologically proven ccRCC lesions were retrospectively collected. 35, 36, 41, and 22 Features were extracted and ranked from PCP, CMP, NP, and three-phase CT images, respectively. The machine learning model based on three-phase CT images [area under the ROC curve (AUC) = 0.87] achieved the best diagnostic performance for differentiating low- from high-grade ccRCC, followed by single-phase NP (AUC = 0.84), CMP (AUC = 0.80), and PCP images (AUC = 0.82).
Conclusion
Machine learning classifiers can be promising noninvasive techniques to differentiate low- and high-Fuhrman nuclear grade ccRCC, and classifiers based on three-phase CT images are superior to those based on features from each single phase.



Similar content being viewed by others
References
C. Global Burden of Disease Cancer, C. Fitzmaurice, D. Dicker, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–527.
R. L. Siegel, K. D. Miller and A. Jemal. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
J. C. Cheville, C. M. Lohse, H. Zincke, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27(5):612–624.
I. S. Gill, E. M. Remer, W. A. Hasan, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173(6):1903–1907.
D. Jocham, A. Richter, L. Hoffmann, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363(9409):594–599.
S. Y. Choi, D. J. Sung, K. S. Yang, et al. Small (< 4 cm) clear cell renal cell carcinoma: correlation between CT findings and histologic grade. Abdom Radiol (NY). 2016;41(6):1160–1169.
C. Chen, Q. Kang, B. Xu, et al. Differentiation of low- and high-grade clear cell renal cell carcinoma: Tumor size versus CT perfusion parameters. Clin Imaging. 2017;46:14–19.
F. Cornelis, E. Tricaud, A. S. Lasserre, et al. Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol. 2015;25(1):24–31.
G. Wu, Z. Zhao, Q. Yao, et al. The Study of Clear Cell Renal Cell Carcinoma with MR Diffusion Kurtosis Tensor Imaging and Its Histopathologic Correlation. Acad Radiol. 2018;25(4):430–438.
B. J. Erickson, P. Korfiatis, Z. Akkus, et al. Machine Learning for Medical Imaging. Radiographics. 2017;37(2):505–515.
L. Zhang, J. Tan, D. Han, et al. From machine learning to deep learning: progress in machine intelligence for rational drug discovery. Drug Discov Today. 2017;22(11):1680–1685.
R. C. Deo. Machine Learning in Medicine. Circulation. 2015;132(20):1920–1930.
B. Kocak, A. H. Yardimci, C. T. Bektas, et al. Textural differences between renal cell carcinoma subtypes: Machine learning-based quantitative computed tomography texture analysis with independent external validation. Eur J Radiol. 2018;107:149–157.
L. Yan, Z. Liu, G. Wang, et al. Angiomyolipoma with minimal fat: differentiation from clear cell renal cell carcinoma and papillary renal cell carcinoma by texture analysis on CT images. Acad Radiol. 2015;22(9):1115–1121.
H. Yu, J. Scalera, M. Khalid, et al. Texture analysis as a radiomic marker for differentiating renal tumors. Abdom Radiol (NY). 2017;42(10):2470–2478.
S. A. Fuhrman, L. C. Lasky and C. Limas. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–663.
P. A. Yushkevich, J. Piven, H. C. Hazlett, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128.
J. J. M. van Griethuysen, A. Fedorov, C. Parmar, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77(21):e104–e107.
A. Zwanenburg, S. Leger, M. Vallières, et al. Image biomarker standardisation initiative. arXiv preprint arXiv:1612.07003. 2016.
A. V. Dorogush, A. Gulin, G. Gusev, et al. Fighting biases with dynamic boosting. arXiv preprint arXiv:1706.09516. 2017.
A. V. Dorogush, V. Ershov and A. Gulin. CatBoost: gradient boosting with categorical features support. arXiv preprint arXiv:1810.11363. 2018.
H. Coy, J. R. Young, M. L. Douek, et al. Association of qualitative and quantitative imaging features on multiphasic multidetector CT with tumor grade in clear cell renal cell carcinoma. Abdom Radiol (NY). 2018.
L. C. Adams, B. Ralla, P. Jurmeister, et al. Native T1 Mapping as an In Vivo Biomarker for the Identification of Higher-Grade Renal Cell Carcinoma: Correlation With Histopathological Findings. Invest Radiol. 2018.
L. Shen, L. Zhou, X. Liu, et al. Comparison of biexponential and monoexponential DWI in evaluation of Fuhrman grading of clear cell renal cell carcinoma. Diagn Interv Radiol. 2017;23(2):100–105.
Y. D. Zhang, C. J. Wu, Q. Wang, et al. Comparison of Utility of Histogram Apparent Diffusion Coefficient and R2* for Differentiation of Low-Grade From High-Grade Clear Cell Renal Cell Carcinoma. AJR Am J Roentgenol. 2015;205(2):W193–201.
A. T. Hale, D. P. Stonko, L. Wang, et al. Machine learning analyses can differentiate meningioma grade by features on magnetic resonance imaging. Neurosurg Focus. 2018;45(5):E4.
Y. W. Park, J. Oh, S. C. You, et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol. 2018.
Y. Li, Z. Qian, K. Xu, et al. MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. Neuroimage Clin. 2018;17:306–311.
C. T. Bektas, B. Kocak, A. H. Yardimci, et al. Clear Cell Renal Cell Carcinoma: Machine Learning-Based Quantitative Computed Tomography Texture Analysis for Prediction of Fuhrman Nuclear Grade. Eur Radiol. 2018.
J. Ding, Z. Xing, Z. Jiang, et al. CT-based radiomic model predicts high grade of clear cell renal cell carcinoma. Eur J Radiol. 2018;103:51–56.
S. Oh, D. J. Sung, K. S. Yang, et al. Correlation of CT imaging features and tumor size with Fuhrman grade of clear cell renal cell carcinoma. Acta Radiol. 2017;58(3):376–384.
J. H. Friedman and B. E. Popescu. Predictive Learning via Rule Ensembles. The Annals of Applied Statistics. 2008;2(3):916–954.
I. Frank, M. L. Blute, J. C. Cheville, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400.
T. Klatte, J. J. Patard, M. de Martino, et al. Tumor size does not predict risk of metastatic disease or prognosis of small renal cell carcinomas. J Urol. 2008;179(5):1719–1726.
L. Marconi, S. Dabestani, T. B. Lam, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol. 2016;69(4):660–673.
R. Guarch, J. M. Cortes, C. H. Lawrie, et al. Multi-site tumor sampling (MSTS) improves the performance of histological detection of intratumor heterogeneity in clear cell renal cell carcinoma (CCRCC). F1000Res. 2016;5:2020.
C. Shen, Z. Liu, M. Guan, et al. 2D and 3D CT Radiomics Features Prognostic Performance Comparison in Non-Small Cell Lung Cancer. Transl Oncol. 2017;10(6):886–894.
J. Ker, L. Wang, J. Rao, et al. Deep learning applications in medical image analysis. IEEE Access. 2018;6:9375–9389.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, F., Cui, EM., Lei, Y. et al. CT-based machine learning model to predict the Fuhrman nuclear grade of clear cell renal cell carcinoma. Abdom Radiol 44, 2528–2534 (2019). https://doi.org/10.1007/s00261-019-01992-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-01992-7