Abstract
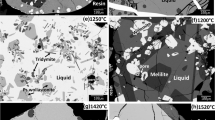
Phase relations in the system CaTiO3-CaSiO3 were experimentally examined at 5.3–14.7 GPa and 1200–1600 °C with a 6–8 type multianvil apparatus. As pressure increases, stability field of perovskite solid solution extends from CaTiO3 to CaSiO3, and the perovskite becomes stable for the entire composition range above about 12.3 GPa. The stability field of Ca(Ti1−X, SiX)2O5 (0.78<x≦1) titanite solid solution +Ca2SiO4 larnite exists in the CaSiO3-rich composition range at 9.3–12.3 GPa and 1200 °C. Perovskite solid solutions containing CaSiO3 component of 0 to 66 mol% could be quenched to 1 atm. The composition-molar volume relationship of perovskite solid solution showed that molar volume of perovskite solid solution linearly reduces from the value of CaTiO3 to that of CaSiO3.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: June 17, 1996 / Revised, accepted: October 22, 1996
Rights and permissions
About this article
Cite this article
Kubo, A., Suzuki, T. & Akaogi, M. High pressure phase equilibria in the system CaTiO3-CaSiO3: stability of perovskite solid solutions. Phys Chem Min 24, 488–494 (1997). https://doi.org/10.1007/s002690050063
Issue Date:
DOI: https://doi.org/10.1007/s002690050063