Abstract
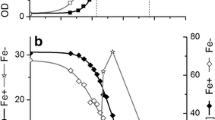
Glycerol and glucose fermentation redox routes by Escherichia coli and their regulation by oxidizing and reducing reagents were investigated at different pHs. Cell growth was followed by decrease of pH and redox potential (E h ). During glycerol utilization at pH 7.5 ∆pH, the difference between initial and end pH, was lower compared with glucose fermentation. After 8 h growth, during glycerol utilization E h dropped down to negative values (−150 mV) but during glucose fermentation it was positive (+50 mV). In case of glycerol H2 was evolved at the middle log phase while during glucose fermentation H2 was produced during early log phase. Furthermore, upon glycerol utilization, oxidizer potassium ferricyanide (1 mM) inhibited both cell growth and H2 formation. Reducing reagents dl-dithiothreitol (3 mM) and dithionite (1 mM) inhibited growth but stimulated H2 production. The findings point out the importance of reductive conditions for glycerol fermentation and H2 production by E. coli.





Similar content being viewed by others
References
Bagramyan K, Trchounian A (1997) Decrease of redox potential in the anaerobic growing Escherichia coli suspension and proton-potassium exchange. Bioelecrochem Bioenerg 43:129–134
Bagramyan K, Galstyan A, Trchounian A (2000) Redox potential is a determinant in the Escherichia coli anaerobic fermentative growth and survival: effects of impermeable oxidant. Bioelectrochemistry 51:151–156
Berrios-Rivera SJ, Sanchez AM, Bennett GN, San KY (2004) Effect of different levels of NADH availability on metabolite distribution in Escherichia coli fermentation in minimal and complex media. Appl Microbiol Biotechnol 65:26–432
Blbulyan S, Avagyan A, Poladyan A, Trchouinian A (2011) Role of Escherichia coli different hydrogenases in H+ efflux and the FOF1-ATPase activity during glycerol fermentation at different pH. Biosci Rep 31:179–184
Booth IR (2005) Module 3.4.3. Glycerol and methylglyoxal metabolism. In: Neidhardt FG (Ed.-in-Chief). Escherichia coli and Salmonella: cellular and molecular biology. Online ed. ASM Press, Washington, DC. http://www.ecosal.org
Brasca M, Morandi R, Lodi R, Tamburini A (2006) Redox potential to discriminate among species of lactic acid bacteria. J Appl Microbiol 103:1516–1524
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Dharmadi Y, Murarka A, Gonzalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnol Bioeng 94:821–828
Gonzalez R, Murarka A, Dharmadi Y, Yazdani S (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10:234–245
Hu H, Wood TK (2010) An evolved Escherichia coli strain for producing hydrogen and ethanol from glycerol. Biochem Biophys Res Commun 391:1033–1038
Khanna S, Goyal A, Moholkar VS (2011) Microbial conversion of glycerol: present status and future prospects. Crit Rev Biotechnol. doi:10.3109/07388551.2011.604839
Kirakosyan G, Bagramyan K, Trchounian A (2004) Redox sensing by Escherichia coli: effects of dithiothreitol, a redox reagent reducing disulphides, on bacterial growth. Biochem Biophys Res Commun 325:803–806
Kwong SCW, Rao G (1992) Effect of reducing agents in anaerobic amino acid fermentation. Biotechnol Bioeng 40:851–857
Lee H, Salerno MB, Rittmann BE (2008) Thermodynamic evaluation on H2 production in glucose fermentation. Environ Sci Technol 42:2401–2407
Michelon D, Abraham S, Ebel B, De Coninck J, Husson F, Feron G, Gervais P (2010) Contribution of exofacial thiol groups in the reducing activity of Lactococcus lactis. FEBS J 277:2282–2290
Murarka A, Dharmadi Y, Yazmandi S, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74:1124–1135
Noguchi K, Riggins DP, Eldahan KG, Kitko RD, Slonczewski JL (2010) Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE 5:e10132
Oblinger JL, Kraft AA (1973) Oxidation–reduction potential and growth of Salmonella and Pseudomonas fluorescens. J Food Sci 38:1108–1112
Oktyabrsky ON, Smirnova GV (1988) Changes in the redox potential and the number of accessible sulfhydryl groups in Escherichia coli and Bacillus subtilis cultures during transient processes. Biokhimia 53:2042–2050 (in Russian)
Ouvry A, Wache Y, Tourdot-Marechal R, Divies C, Cachon R (2002) Effects of oxidoreduction potential combined with acetic acid, NaCl and temperature on growth, acidification, and membrane properties of Lactobacillus plantarum. FEMS Microbiol Lett 214:257–261
Piskarev M, Ushkanov VA, Aristova NA, Likhachev PP, Myslivets TS (2010) Establishment of the redox potential of water saturated with hydrogen. Biophysics 55:19–24
Poladyan A, Trchounian A (2009) Production of molecular hydrogen by mixed-acid fermentation in bacteria and its energetic. In: Trchounian A (ed) Bacterial membranes ultrastructure, bioelectrochemistry, bioenergetics and biophysics. Research Signpost, Trivandrum, pp 197–231
Repaske R, Clayton MA (1978) Control of Escherichia coli growth by CO2. J Bacteriol 135:1162–1164
Riondet C, Cachon R, Waché Y, Alcaraz G, Divies C (2000) Extracellular oxidoreduction potential modifies carbon and electron flow in Escherichia coli. J Bacteriol 182:620–624
Sawers RG (2005) Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans 33:42–46
Soghomonyan D, Akopyan K, Trchounian A (2011) pH and oxidation–reduction potential change of environment during growth of lactic acid bacteria: effect of oxidizers and reducers. Appl Biochem Microbiol 47:33–38
Trchounian A (2004) Escherichia coli proton-translocating F 0 F 1-ATP synthase and its association with solute secondary transpopters and/or enzymes of anaerobic oxidation–reduction under fermentation. Biochem Biophys Res Commun 315:1051–1057
Trchounian A, Kobayashi H (1999) Kup is the major K+ uptake system in Escherichia coli upon hyper-osmotic stress at a low pH. FEBS Lett 447:144–148
Trchounian K, Trchounian A (2009) Hydrogenase 2 is most and hydrogenase 1 is less responsible for H2 production by Escherichia coli under glycerol fermentation at neutral and slightly alkaline pH. Int J Hydrogen Energy 34:8839–8845
Trchounian K, Sanchez-Torres V, Wood TK, Trchounian A (2011) Escherichia coli hydrogenase activity and H2 production under glycerol fermentation at low pH. Int J Hydrogen Energy 36:4323–4331
Trchounian K, Poladyan A, Vassilian A, Trchounian A (2012) Multiple and reversible hydrogenases for hydrogen production by Escherichia coli: dependence on fermentation substrate, pH and FOF1-ATPase. Crit Rev Biochem Mol Biol 47(3):236–249
Vassilian A, Trchounian A (2009) Environment oxidation–reduction potential and redox sensing by bacteria. In: Trchounian A (ed) Bacterial membranes ultrastructure, bioelectrochemistry, bioenergetics and biophysics. Research Signpost, Trivandrum, pp 163–195
Waché Y, Riondet C, Diviès C, Cachon R (2002) Effect of reducing agents on the acidification capacity and the proton motive force of Lactococcus lactis ssp. cremoris resting cells. Bioelectrochemistry 57:113–118
Warden WC, Hentgen DJ (1975) Differential effects of oxygen and oxidation–reduction potential on the multiplication of three species of anaerobic intestinal bacteria. J Appl Microbiol 30:781–785
Yazdani S, Gonzalez R (2007) Anaerobic fermentation of glycerol, a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–221
Acknowledgments
This study was done in frame of Research Grant to AT (#11-F-202) from Ministry of Education and Sciences of the Republic of Armenia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poladyan, A., Avagyan, A., Vassilian, A. et al. Oxidative and Reductive Routes of Glycerol and Glucose Fermentation by Escherichia coli Batch Cultures and Their Regulation by Oxidizing and Reducing Reagents at Different pHs. Curr Microbiol 66, 49–55 (2013). https://doi.org/10.1007/s00284-012-0240-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0240-2