Abstract
Key message
Salicylic acid-signaling pathway and ethylene biosynthesis were induced in tomato treated with Trichoderma harzianum when infected by root-knot nematodes and limited the infection by activation of SAR and ethylene production.
Abstract
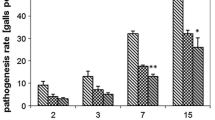
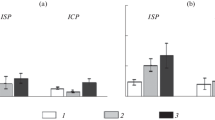
Soil pre-treatment with Trichoderma harzianum (Th) strains ITEM 908 (T908) and T908-5 decreased susceptibility of tomato to Meloidogyne incognita, as assessed by restriction in nematode reproduction and development. The effect of T. harzianum treatments on plant defense was detected by monitoring the expression of the genes PR-1/PR-5 and JERF3/ACO, markers of the SA- and JA/ET-dependent signaling pathways, respectively. The compatible nematode–plant interaction in absence of fungi caused a marked suppression of PR-1, PR-5, and ACO gene expressions, either locally or systemically, whilst expression of JERF3 gene resulted unaffected. Conversely, when plants were pre-treated with Th-strains, over-expression of PR-1, PR-5, and ACO genes was observed in roots 5 days after nematode inoculation. JERF3 gene expression did not change in Th-colonized plants challenged with nematodes. In the absence of nematodes, Trichoderma-root interaction was characterized by the inhibition of both SA-dependent signaling pathway and ET biosynthesis, and, in the case of PR-1 and ACO genes, this inhibition was systemic. JERF3 gene expression was systemically restricted only at the very early stages of plant–fungi interaction. Data presented indicate that Th-colonization primed roots for Systemic Acquired Resistance (SAR) against root-knot nematodes and reacted to nematode infection more efficiently than untreated plants. Such a response probably involves also activation of ET production, through an augmented transcription of the ACO gene, which encodes for the enzyme catalyzing the last step of ET biosynthesis. JA signaling and Induced Systemic Resistance (ISR) do not seem to be involved in the biocontrol action of the tested Th-strains against RKNs.






Similar content being viewed by others
References
Alfano G, Ivey MLL, Cakir C, Bos JIB, Miller SA, Madden LV, Kamoun S, Hoitink HAJ (2007) Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology 97:429–437
Bekal S, Niblack TL, Lambert KNA (2003) A chorismate mutase from the soybean cyst nematode Heteroderaglycines shows polymorphisms that correlate with virulence. Mol Plant Microbe In 16:439–446
Derksen H, Rampitsch C, Daayf F (2013) Signaling cross-talk in plant disease resistance. Plant Sci 207:79–87
Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kennerley CM, Monte E, Mukherjee P K, Zeilinger S, Grigoriev IV, Kubicek CP (2011) Trichoderma: the genomics of opportunistic success. Nature Rev 9:749–759
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:85–209
Fudali SL, Wang C, Williamson VM (2013) Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol Plant Microbe In 26:75–86
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Graeme-Cook KA, Faull JL (1991) Effect of ultraviolet-induced mutants of Trichoderma harzianum with altered antibiotic production on selected pathogens in vitro. Can J Microbiol 37:659–664
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species—opportunistic avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hermosa R, Viterbo A, Chet I, Monte E (2012) Plant beneficial effects of Trichoderma and of its genes. Microbiology 158:17–25
Kabaluk T, Gazdik K (2005) Directory of microbial pesticides for agricultural crops in OECD Countries. Revised September 2005. Agriculture and Agri-Food Canada, 1–242
Kim YS, Choi D, Lee MM, Lee SH, Kim WT (1998) Biotic and abiotic stress-related expression of ACO gene family in Nicotiana glutinosa. Plant Cell Physiol 39:565–573
Leonetti P, Costanza A, Zonno M, Molinari S, Altomare C (2014) How fungi interact with nematode to activate the plant defence response in tomato plants. Agric Appl Biol Sci 79(3):357–363
Li R, Rashotte AM, Singh NK, Lawrence KS, Weaver DB, Locy RD (2014) Integrated signaling networks in plant response to sedentary endoparasitic nematodes: a perspective. Plant Cell Rep 46(4):365–375
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Marzano M, Gallo A, Altomare C (2013) Improvement of biocontrol efficacy of Trichoderma harzianum vs. Fusarium oxysporum f. sp. lycopersici through UV-induced tolerance to fusaric acid. Biol Control 67:397–408
Mathys J, De Cremer K, Timmermans P, Van Kerckhove S, Lievens B, Vanhaecke M, Cammue BPA, De Coninck B (2012) Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatumT-382 against Botrytis cinerea infection. Front Plant Sci 3:1–25
Molinari S (2009) Bioassays on plant-nematode interactions. In: Narwal SS, Sampietro DA, Catalàn CAN, Vattuone MA, Politycka B (eds) Plant Bioassays. Science Publisher, Enfield, pp 293–326
Molinari S (2011) Natural genetic and induced plant resistance, as a control strategy to plant-parasitic nematodes alternative to pesticides. Plant Cell Rep 30:311–323
Molinari S (2015) Immune responses induced by salicylic and jasmonic acids against plant parasites. In: Santos P (ed) Salicylic acid and jasmonic acid: biosynthesis, functions and role in plant development. Nova Science Publishers Inc., New York, pp 1–35
Molinari S (2016) Systemic acquired resistance activation in solanaceous crops as a management strategy against root-knot nematodes. Pest Manag Sci 72:888–896
Molinari S, Abd-Elgawad MM (2007) Catalase inhibition as a biochemical marker of resistance to root-knot nematodes in tomato. Nematol medit 35:237–242
Molinari S, Fanelli E, Leonetti P (2014) Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol Plant Pathol 15:255–264
Nawrocka J, Małolepsa U (2013) Diversity in plant resistance induced by Trichoderma. Biol Control 67:14–156
Portillo M, Cabrera J, Lindsey K, Topping J et al (2013) Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: a functional role for gene repression. New Phytol 197:1276–1290
Pozo MJ, Azcón-Aguilar C (2007) Unravelling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Selim ME, Mahdy ME, Sorial ME, Dababat AA, Sikora RA (2014) Biological and chemical dependent systemic resistance and their significance for the control of root-knot nematodes. Nematology 16:917–927
Sharon E, Bar-Eyal M, Chet I, Herrera-Estrella A, Kleifeld, Spiegel Y (2001) Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopathology 91:687–693
Sharon E, Chet I, Viterbo A, Bar-Eyal M, Nagan H, Samuels GJ, Spiegel Y (2007) Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur J Plant Pathol 118:247–258
Sharon E, Chet I, Bar-Eyal M (2009) Biocontrol of root-knot nematodes by Trichoderma—modes of action. Proc. IOBC Meeting on Multitrophic Interactions in Soil, Dijon, France, IOBC/WPRS Bulletin 42:159–163
Shoresh M, Yedidia I, Chet I (2005) Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95:76–84
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 48:21–43
Sticher L, Mauch-Mani B, Métraux JP (1997) Systemic acquired resistance. Annu Rev Phytopathol 35:235–270
Stirling GR (2011) Biological control of plant-parasitic nematodes: an ecological perspective, a review of progress and opportunities for further research. In Davies K, Spiegel Y (eds) Biological control of plant-parasitic nematodes: building coherence between microbial ecology and molecular mechanisms. Progress in biological control 11, Springer Science + Business Media B.V., 1–38
Szabó M, Csepregi K, Gálber M, Virányi F, Fekete C (2012) Control plant-parasitic nematodes with Trichoderma species and nematode-trapping fungi: The role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biol Control 63:121–128
Ton J, Van Pelt JA, Van Loon LC, Pieterse CMJ (2002). Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol Plant Microbe In 15:27–34
Trudgill DL, Blok VC (2001) Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol 39:53–77
Uehara T, Sugiyama S, Matsura H, Arie T, Masuta C (2010) Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol 51:1524–1536
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Wang H, Huang Z, Chen Q, Zhang Z, Zhang H, Wu Y, Huang D, Huang R (2004) Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol Biol 55:183–192
Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Lombardi N, Pascale A, Lanzuise S, Manganiello G, Lorito M (2014) Trichoderma-based products and their widespread use in agriculture. Open Mycol J 8:127–139
Yan C, Xie D (2015) Jasmonate in plant defence: sentinel or double agent? Plant Biotech J 13:1233–1240
Yoshioka Y, Ichikawa H, Naznin HA, Kogure A, Hyakumachi M (2011) Systemic resistance induced in Arabidopsis thaliana by Trichoderma asperellum SKT-1, a microbial pesticide of seedborne diseases of rice. Pest Manag Sci 68:60–66
Acknowledgements
The work of P. Leonetti and S. Molinari was carried out within the sub-project OR3 SOS-POM of the project CISIA, funded by the National Research Council (CNR) of Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Kathryn K. Kamo.
Rights and permissions
About this article
Cite this article
Leonetti, P., Zonno, M.C., Molinari, S. et al. Induction of SA-signaling pathway and ethylene biosynthesis in Trichoderma harzianum-treated tomato plants after infection of the root-knot nematode Meloidogyne incognita . Plant Cell Rep 36, 621–631 (2017). https://doi.org/10.1007/s00299-017-2109-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2109-0