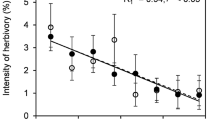
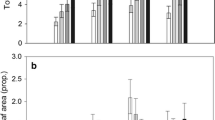
Abstract
Arctic plants and herbivores are subject to ongoing climatic changes that are more rapid and extreme than elsewhere on the planet, and thus it is pivotal to understand the arctic plant-herbivore interactions in a global change context. We examined how infestation by an eriophyoid gall mite affects the circumpolar shrub Salix arctica, and how the effects vary across vegetation types. Specifically, we compared multiple leaf characteristics (leaf area, biomass, nutrient levels, δ15N and δ13C, and stress and performance of the photosynthetic apparatus) of infested leaves to those of un-infested leaves. Furthermore, we examined how altered environmental conditions, here experimentally manipulated levels of temperature, water and nutrients, shading, and UV-B radiation, affect the prevalence, density, and intensity of gall mite infestation and its impacts on S. arctica. Infested leaves were smaller in area and biomass and had lower nitrogen and carbon pools. However, their carbon concentration was higher, possibly because the galls acted as carbon sinks. The smaller photosynthetic area and lower nutrient content caused increased stress on the photosynthetic apparatus in infested leaves. The remaining leaf tissue responded with a higher photosynthetic performance, although there were indications of a general reduction in photosynthesis. Female leaves were more affected than male leaves. The experimental manipulations of environmental conditions did not affect the gall prevalence, density, or intensity on S. arctica leaves. Rather, plants responded positively to the treatments, reducing the effects of the galls to in-significance. This suggests a higher tolerance and defense against gall mites under future climate conditions.




Similar content being viewed by others
References
Abrahamsen WG, McCrea KD (1986) Nutrient and biomass allocation in Solidago altissima: effects of two stem gallmakers, fertilization, and ramet isolation. Oecologia 68:174–180
Albert KR, Ro-Poulsen H, Mikkelsen TN, Bredahl L, Haakansson KB (2004) Effects of reducing the ambient UV-B radiation in the high Arctic on Salix arctica and Vaccinium uliginosum. Phyton 45:41–49
Albert KR, Mikkelsen TN, Ro-Poulsen H, Arndal MF, Michelsen A (2011) Ambient UV-B radiation reduces PSII performance and net photosynthesis in high Arctic Salix arctica. Environ Exp Bot 73:10–18
Amrine JW, Stasny TA (1994) Catalog of the eriophyoidea (Acarina: Prostigmata) of the world. Indira Publish. House, West Bloomfield
Argus GW, McJannet CL, Dallwitz MJ (1999) Salicaceae of the Canadian Arctic Archipelago: descriptions, illustrations, identification, and information retrieval. Version 2: 2nd November 2000. www.mun.ca/biology/delta/arcticf/. Accessed March 2012
Bay C (1998) Vegetation mapping of Zackenberg Valley, Northeast Greenland. Danish Polar Center & Botanical museum, University of Copenhagen, Denmark
Berg TB, Schmidt NM, Høye TT, Aastrup P, Hendrichsen DK, Forchhammer MC, Klein DR (2008) High-Arctic plant-herbivore interactions under climate influence. Adv Ecol Res 40:275–298
Böcher J (2001) Insekter og andre smådyr-i Grønlands fjeld og ferskvand. Atuagkat, Vojens
Boecklen WJ, Price PW, Mopper S (1990) Sex and drugs and herbivores: sex-biased herbivory in arroyo willow (Salix Lasiolepis). Ecology 71:581–588
Bryant JP, Reichardt PB (1992) Controls over secondary metabolite production by Arctic woody plants. In: Chapin FS, Jefferies RL, Reynold JF, Shaver G, Svoboda J (eds) Arctic ecosystems in a changing climate: an ecophysiological perspective. Academic Press, London, pp 377–390
Callaghan TV, Carlsson BA, Tyler NJC (1989) Historical records of climate-related growth in Cassiope tetragona from the Arctic. J Ecol 77:823–837
Callaghan TV, Jonasson S, Nichols H, Heywood RB, Wookey PA (1995) Arctic terrestrial ecosystems and environmental change [and discussion]. Phil Trans R Soc Lond A 352:259–276
Chapin FS, Matson PA, Mooney HA (2002a) Carbon input to terrestrial ecosystems. In: Principles of terrestrial ecosystem ecology. Springer, New York, pp 97–122
Chapin FS, Matson PA, Mooney HA (2002b) Terrestrial plant nutrient use. In: Principles of terrestrial ecosystem ecology. Springer, New York, pp 176–196
Christiansen CT, Svendsen SH, Schmidt NM, Michelsen A (2012) High arctic heath soil respiration and biogeochemical dynamics during summer and autumn freeze-in—effects of long term enhanced water and nutrient supply. Glob Change Biol 18:3224–3236
Collet DM (2004) Willows of interior Alaska. US Fish and Wildlife Service, USA
Conn EE (1984) Compartmentation of secondary compounds. Ann Proc Phytochem Soc Eur 24:1–28
Cooper EJ, Wookey PA (2003) Floral herbivory of Dryas octopetala by Svalbard reindeer. Arct Antarct Alp Res 35:369–376
Cornelissen T, Stiling P (2005) Sex-biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos 111:488–500
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Penuelas J, Reich PB, Schuur EAG, Stock WD, Templer PH, Virginia RA, Welker JM, Wright IJ (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992
Egan SP, Ott JR (2007) Host plant quality and local adaptation determine the distribution of a gall-forming herbivore. Ecology 88:2868–2879
Elberling B, Tamstorf MP, Michelsen A, Arndal MF, Sigsgaard C, Illeris L, Bay C, Hansen BU, Christensen TR, Hansen ES, Jakobsen BH, Beyens L (2008) Soil and plant community-characteristics and dynamics at Zackenberg. Adv Ecol Res 40:223–248
Ellebjerg SM, Tamstorf MP, Illeris L, Michelsen A, Hansen BU (2008) Inter-annual variability and controls of plant phenology and productivity at Zackenberg. Adv Ecol Res 40:249–273
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics, 2.2nd edn. Sinauer Associates, Inc., Sunderland
Graglia E, Jonasson S, Michelsen A, Schmidt IK (1997) Effects of shading, nutrient application and warming on leaf growth and shoot densities of dwarf shrubs in two arctic-alpine plant communities. Ecoscience 4:191–198
Gripenberg S, Roslin T (2005) Host plants as islands: resource quality and spatial setting as determinants of insect distribution. Ann Zool Fennici 42:335–345
Hakkarainen H, Roininen H, Virtanen R (2005) Negative impact of leaf gallers on arctic-alpine dwarf willow, Salix herbacea. Polar Biol 28:647–651
Hansen BU, Sigsgaard C, Rasmussen L, Cappelen J, Hinkler J, Mernild SH, Petersen D, Tamstorf MP, Rasch M, Hasholt B (2008a) Present-day climate at Zackenberg. Adv Ecol Res 40:111–149
Hansen J, Hansen LH, Tamstorf MP, Christoffersen K, Jeppesen E, Schmidt NM (2008b) Zackenberg basic: the BioBasic programme. Zackenberg ecological research operations, 13th annual report, 2007. Copenhagen, Danish Polar Center, Danish Agency for Science, Technology and Innovation, Ministry of Science, Technology and Innovation, 2008
Hartley SE (1998) The chemical composition of plant galls: are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 113:492–501
Haukioja E (1981) Invertebrate herbivory at tundra sites. In: Bliss LC, Heal OW, Moore JJ (eds) Tundra ecosystems. Cambridge University Press, Cambridge, pp 547–556
Hodkinson ID, Webb NR, Bale JS, Block W, Coulson JC, Strathdee AT (1998) Global change and Arctic ecosystems: conclusions and predictions from experiments with terrestrial invertebrates on Spitsbergen. Arct Alp Res 30:306–313
Illeris L, Michelsen A, Jonasson S (2003) Soil plus root respiration and microbial biomass following water, nitrogen, and phosphorus application at a high arctic semi desert. Biogeochemistry 65:15–59
Jefferies RL, Klein CJ, Shaver G (1994) Vertebrate herbivores and northern plant communities: reciprocal influences and responses. Oikos 71:193–206
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77:257–264. doi:10.1111/j.1365-2656.2007.01339.x
Klein DR, Bay C (1991) Diet selection by vertebrate herbivores in the high arctic of Greenland. Holarctic Ecol 14:152–155
Kristensen DK, Kristensen E, Forchhammer MC, Michelsen A, Schmidt NM (2011) Arctic herbivore diet can be inferred from stable carbon and nitrogen isotopes in C3 plants, faeces, and wool. Can J Zool 89:893–900
Kuczynski L, Skoracka A (2005) Spatial distribution of galls caused by Aculus tetanothrix (Acari: Eriophyoidea) on arctic willows. Exp Appl Acarol 36:277–289. doi:10.1007/s10493-005-7551-y
Kukal O, Dawson TE (1989) Temperature and food quality influences feeding behavior, assimilation efficiency and growth rate of arctic woolly-bear caterpillars. Oecologia 79:526–532
Larson KC (1998) The impact of two gall-forming arthropods on the photosynthetic rates of their hosts. Oecologia 115:161–166
Larson KC, Whitham TG (1991) Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia 88:15–21
Larsson SA, Wiren A, Lundgren L, Ericsson T (1986) Effects of light and nutrient stress on leaf phenolic chemistry in Salix dasyclados and susceptibility to Galerucella lineola. Oikos 47:205–210
Leite GLD, Picanco M, Zanuncio JC, Marquini F (2003) Factors affecting mite herbivory on eggplants in Brazil. Exp Appl Acarol 31:243–252
Lindquist EE, Sabelis MW, Bruin J (eds) (1996) World crop pests 6: eriophyoid mites. Their biology, natural enemies and control, vol 6. Elsevier Science, Amsterdam
MacLean SFJ (1981) Fauna of tundra ecosystems: invertebrates. In: Bliss LC, Heal OW, Moore JJ (eds) Tundra ecosystems. Cambridge University Press, Cambridge, pp 509–516
Marshall VG, Clayton MR (2004) Biology and phenology of Cecidophyopsis psilaspis (Acari: Eriophyidae) on Pacific yew (Taxaceae). Can Entomol 136:695–710
Marshall JD, Brooks JR, Lajtha K (2007) Sources of variation in the stable isotopic composition of plants. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell publishing, Oxford
Massad TJ, Dyer LA (2010) A meta-analysis of the effects of global environmental change on plant-herbivore interactions. Arthropod Plant Interact 4:181–188
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Mazza CA, Zavala J, Scopel AL, Ballare CL (1999) Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. P Natl Acad Sci USA 96:980–985
McCrea KD, Abrahamsen WG, Weis AE (1985) Goldenrod ball gall effects on Solidago altissima. Ecology 66:1902–1907
Meyer GA (2000) Effects of insect feeding on growth and fitness of goldenrod (Solidago altissima). Recent Res Devel Entomol 3:29–41
Michalska K, Skoracka A, Navia D, Amrine JW (2010) Behavioural studies on eriophyoid mites: an overview. Exp Appl Acarol 51:31–59
Michelsen A, Quarmby C, Sleep D, Jonasson S (1998) Vascular plant 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 115:406–418
Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias-Fauria M, Sass-Klaassen U, Lévesque E, Boudreau S, Ropars P, Hermanutz L, Trant A, Collier LS, Weijers S, Rozema J, Rayback SA, Schmidt NM, Schaepman-Strub G, Wipf S, Rixen C, Ménard C, Venn S, Goetz S, Andreau-Hayles L, Elmendorf SC, Ravolainen V, Welker J, Grogan P, Epstein HE, Hik DS (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509
Nykänen H, Koricheva J (2004) Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta analysis. Oikos 104:247–268
Olofsson J, Hulme PE, Oksanen L, Suominen O (2004) Importance of large and small mammalian herbivores for the plant community structure in the forest tundra ecotone. Oikos 106:324–334
Ozman SK, Goolsby JA (2005) Biology and phenology of the eriophyid mite, Floracarus perrepae, on its native host in Australia, Old World climbing fern, Lygodium microphyllum. Exp Appl Acarol 35:197–213
Parsons AN, Welker JM, Wookey PA, Press MC, Callaghan TV, Lee JA (1994) Growth responses of four sub-arctic dwarf shrubs to simulated environmental change. J Ecol 82:307–318
Patankar R, Thomas SC, Smith SM (2011) A gall-inducing arthropod drives declines in canopy tree photosynthesis. Oecologia 167:701–709
Patankar R, Starr G, Mortazavi B, Oberbauer SF, Rosenblum A (2013) The effects of mite galling on the ecophysiology of two arctic willows. Arct Antarct Alp Res 45:1–8. doi:10.1657/1938-4246-1645.1651
Petanovic R, Kielkiewicz M (2010) Plant-eriophyoid mite interactions: cellular biochemistry and metabolic responses induced in mite-injured plants. Part I. Exp Appl Acarol 51:61–80. doi:10.1007/s10493-010-9351-2
Post E, Pedersen C (2008) Opposing plant community responses to warming with and without herbivores. PNAS 105:12353–12358
Quiros-Gonzalez M (2000) Phytophagous mite populations on Tahiti lime, Citrus latifolia, under induced drought conditions. Exp Appl Acarol 24:897–904
Raven P (1983) Phytophages of xylem and phloem. Rec Adv Ecol 14:136–234
Richardson SJ, Press MC, Parsons AN, Hartley SE (2002) How do nutrients and warming impact on plant communities and their insect herbivores? A 9-year study from a sub-Arctic heath. J Ecol 90:544–556
Rosenqvist E, Van Kooten O (2003) Chlorophyll fluorescence: a general description and nomenclature. In: Dell JR, Toivonen PMA (eds) Practical applications of chlorophyll fluorescence in plant biology. Kluwer academic Publishers, Dordrecht
Rousseaux MC, Julkunen-Tiitto R, Searles PS, Scopel AL, Aphalo PJ, Ballare CL (2004) Solar UV-B radiation affects leaf quality and insect herbivory in the southern beech tree Nothofagus antarctica. Oecologia 138:505–512. doi:10.1007/s00442-003-1471-5
Schmidt NM, Baittinger C, Forchhammer MC (2006) Reconstructing century-long snow regimes using estimates of high Arctic Salix arctica radial growth. Arct Antarct Alp Res 38:257–262
Schmidt NM, Hansen LH, Hansen J, Berg TB, Meltofte H (2012) BioBasis manual: conceptual design and sampling procedures of the biological monitoring programme within Zackenberg basic, 15th edn. Aarhus University, Department of Bioscience, Roskilde
Schultz BB (1992) Insect herbivores as potential causes of mortality and adaptation in gallforming insects. Oecologia 90:297–299
Skoracka A, Kuczynski L (2003) Population dynamics of eriophyoid mites (Acari: Eriophyoidea) living on grasses in Poland. Biol Lett 40:101–110
Stinner BR, Abrahamsen WG (1979) Energetics of the Solidago Canadensis-stem gall insect-parasitoid guild interactions. Ecology 60:918–926
Strong DR, Lawton JH, Southwood SR (1984) Insects on plants. Community patterns and mechanisms. Harvard University Press, Cambridge, MA
Szydlo W, Skaftason JF, Skoracka A (2010) Eriophyoid mites (Prostigmata: Eriophyoidea: Eriophyidae) from Icleand: one new species, and three new mite records. Ann Zool 60:139–157. doi:10.3161/000345410x499623
Taper ML, Case TJ (1987) Interactions between oak tannins and parasite community structure: unexpected benefits of tannins to cynipid gall-wasps. Oecologia 71:254–261
Taper ML, Zimmermann EM, Case TJ (1986) Sources of mortality for a cynipid gall-wasp (Dryocosmus dubiosus (Hymenoptera: Cynipidae)): the importance of the tannin/fungus interaction. Oecologia 68:437–495
Trumble JT, Kolodny-Hirsch DM, Ting IP (1993) Plant compensation for arthropod herbivory. Annu Rev Entomol 38:93–119
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11:1351–1363
US Forest Service (2012) Salix Arctica Pallas. www.fs.fed.us/global/iitf/pdf/shrubs/Salix%20arctica.pdf. Accessed March 2012
Van Leeuwen T, Witters J, Nauen R, Duso C, Tirry L (2010) The control of eriophyoid mites: state of the art and future challenges. Exp Appl Acarol 51:205–224. doi:10.1007/s10493-009-9312-9
Varadarajan MK, David PMM (2002) Population dynamics of the coconut mite Aceria guerreronis Keifer (Acari: Eriophyidae) and associated arthropods in Tamil Nadu, India. Insect Sci Its Appl 22:47–59
Vuorisalo T, Walls M, Kuitunen H (1990) Gall mite (Eriophyes laevis) infestation and leaf removal affect growth of leaf area in black alder (Alnus glutinosa) short shoots. Oecologia 84:122–125
Wilf P, Labandeira CC (1999) Response of plant-insect associations to paleocene-eocene warming. Science 284:2153–2156
Wookey PA, Aerts R, Bardgett RD, Baptist F, BrÅThen KA, Cornelissen JHC, Gough L, Hartley IP, Hopkins DW, Lavorel S, Shaver GR (2009) Ecosystem feedbacks and cascade processes: understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob Change Biol 15:1153–1172. doi:10.1111/j.1365-2486.2008.01801.x
Yano Y, Shaver GR, Giblin AE, Rastetter EB (2010) Depleted 15N in hydrolysable-N of arctic soils and its implication for mycorrhizal fungi–plant interaction. Biogeochemistry 97:183–194
Zavala JA, Scopel AL, Ballare CL (2001) Effects of ambient UV-B radiation on soybean crops: impact on leaf herbivory by Anticarsia gemmatalis. Plant Ecol 156:121–130
Acknowledgments
Special thanks are due to the Zackenberg Research Station for the logistic support. We also thank the Danish National Research Foundation for supporting the activities within the Center for Permafrost (CENPERM DNRF100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mosbacher, J.B., Schmidt, N.M. & Michelsen, A. Impacts of eriophyoid gall mites on arctic willow in a rapidly changing Arctic. Polar Biol 36, 1735–1748 (2013). https://doi.org/10.1007/s00300-013-1393-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1393-6