Abstract
Objectives
The development of integrated magnetic resonance (MR)-positron emission tomography (PET) hybrid imaging opens up new horizons for imaging in neuro-oncology. In cerebral gliomas the definition of tumour extent may be difficult to ascertain using standard MR imaging (MRI) only. The differentiation of post-therapeutic scar tissue, tumour rests and tumour recurrence is challenging. The relationship to structures such as the pyramidal tract to the tumour mass influences the therapeutic neurosurgical approach.
Methods
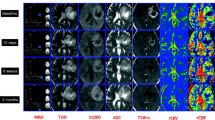
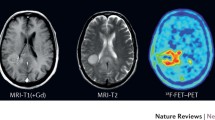
The diagnostic information may be enriched by sophisticated MR techniques such as diffusion tensor imaging (DTI), multiple-volume proton MR spectroscopic imaging (MRSI) and functional MRI (fMRI). Metabolic imaging with PET, especially using amino acid tracers such as 18F-fluoroethyl-l-tyrosine (FET) or 11C-l-methionine (MET) will indicate tumour extent and response to treatment.
Results
The new technologies comprising MR-PET hybrid systems have the advantage of providing comprehensive answers by a one-stop-job of 40-50 min. The combined approach provides data of different modalities using the same iso-centre, resulting in optimal spatial and temporal realignment. All images are acquired exactly under the same physiological conditions.
Conclusions
We describe the imaging protocol in detail and provide patient examples for the different imaging modalities such as FET-PET, standard structural imaging (T1-weighted, T2-weighted, T1-weighted contrast agent enhanced), DTI, MRSI and fMRI.
Key Points
• Hybrid MR-PET opens up new horizons in neuroimaging
• Hybrid MR-PET allows brain tumour assessment in one stop
• Hybrid MR-PET allows simultaneous acquisition of structural, functional and molecular images








Similar content being viewed by others
References
Weller M, Wick W (2003) Primäre intrakranielle und spinale Tumore. In: Brandt T, Dichgans J, Diener HC (eds) Therapie und Verlauf neurologischer Erkrankungen, 4th edn. Kohlhaas, Berlin, pp 776–812
Julia-Sape M, Coronel I, Majos C, et al (2011) Prospective diagnostic performance evaluation of single-voxel 1H MRS for typing and grading of brain tumours. NMR Biomed 25:661–673
Pauleit D, Stoffels G, Bachofner A et al (2009) Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol 36:779–787
Heiss WD, Raab P, Lanfermann H (2011) Multimodality assessment of brain tumors and tumor recurrence. J Nucl Med 52:1585–1600
Fan GG, Deng QL, Wu ZH, Guo QY (2006) Usefulness of diffusion/perfusion-weighted MRI in patients with non-enhancing supratentorial brain gliomas: a valuable tool to predict tumour grading? Br J Radiol 79:652–658
Majos C, Julia-Sape M, Alonso J et al (2004) Brain tumor classification by proton MR spectroscopy: comparison of diagnostic accuracy at short and long TE. AJNR Am J Neuroradiol 25:1696–1704
Widhalm G, Krssak M, Minchev G et al (2011) Value of 1H-magnetic resonance spectroscopy chemical shift imaging for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast-enhancement. J Neurol Neurosurg Psychiatry 82:512–520
Basser PJ (1995) Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8:333–344
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 15:456–467
Bick AS, Mayer A, Levin N (2011) From research to clinical practice: Implementation of functional magnetic imaging and white matter tractography in the clinical environment. J Neurol Sci 312:158–165
Boss A, Kolb A, Hofmann M et al (2010) Diffusion tensor imaging in a human PET/MR hybrid system. Investig Radiol 45:270–274
Sauter AW, Wehrl HF, Kolb A, Judenhofer MS, Pichler BJ (2010) Combined PET/MRI: one step further in multimodality imaging. Trends Mol Med 16:508–515
Schiepers C, Dahlbom M (2011) Molecular imaging in oncology: the acceptance of PET/CT and the emergence of MR/PET imaging. Eur Radiol 21:548–554
Herzog H, Langen KH, Kaffanke J et al (2010) MR-PET opens new horizons in neuroimaging. Futur Neurol 5:807–815
Herzog H, Pietrzyk U, Shah NJ, Ziemons K (2010) The current state, challenges and perspectives of MR-PET. NeuroImage 49:2072–2082
Herzog H, Langen KJ, Weirich C et al (2011) High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 50:74–82
de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA (2007) Performance evaluation of the ECAT HRRT: an LSO-LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol 52:1505–1526
Rota Kops E, Herzog, H (2008) Template based attenuation correction for PET in MR-PET scanners. IEEE Nuclear Sciences Symposium Conference Record. IEEE, New York, pp 3786–3789
Ashburner J, Friston KJ (1999) Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7:254–266
Michel CL, Sanabria S (1999) Weighted schemes applied to 3D-OSEM reconstruction in PET. IEEE Nuclear Sciences Symposium Conference Record. IEEE, New York, pp 1152–1157.
Scheins JJ, Herzog H, Shah NJ (2011) Fully-3D PET image reconstruction using scanner-independent, adaptive projection data and highly rotation-symmetric voxel assemblies. IEEE Trans Med Imaging 30:879–892
Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Todd Constable R (2009) Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia 50:2225–2241
Stippich C, Rapps N, Dreyhaupt J et al (2007) Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology 243:828–836
Sala-Llonch R, Pena-Gomez C, Arenaza-Urquijo EM et al (2011) Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex, doi:10.1016/j.cortex.2011.07.006
Langen KJ, Bartenstein P, Boecker H et al (2011) German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids. Nuklearmedizin 50:167–173
Morita N, Harada M, Otsuka H, Melhem ER, Nishitani H (2011) Clinical application of MR spectroscopy and imaging of brain tumor. Magn Reson Med Sci 9:167–175
Schlemmer HP, Pichler BJ, Schmand M et al (2008) Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology 248:1028–1035
Acknowledgements
We thank Susanne Schaden and Cornelia Frey for their excellent technical assistance. The INM-4 is supported by the German Ministry of Education and Research/Bundesministerium für Bildung und Forschung (BMBF), and Siemens, Germany. Implementation of fMRI and DTI was in part supported by the Deutsche Forschungsgemeinschaft grant DFG SH 79/2-2.
Financial disclosure
Dr. Neuner, Dr. Kaffanke, Prof. Langen, Dr. Rota Kops, Mr. Tellmann, Dr. Stoffels, Mr. Weirich, Dr. Filss, Dr. Scheins, Prof. Herzog have no financial conflicts of interest to report. Prof. Shah has received research grants and scientific support from the German Ministry of Education and Research/Bundesministerium für Bildung und Forschung (BMBF) and Siemens, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Neuner and J. B. Kaffanke contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
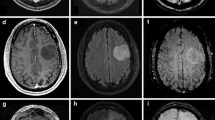
Case 1. Simultaneously acquired data include structural anatomical data T1-weighted (top row) and FET-PET data (bottom row). The PET data show an inhomogeneous tracer uptake in the corpus callosum and cingulate gyrus. In the centre of the tumour there is no tracer uptake due to central necrosis (PNG 325 kb)
ESM Fig. 2
Case 2. Simultaneously acquired data include structural anatomical data T1-weighted (row 1 from top) and FET-PET data (bottom row). PET data demonstrate a pathological tracer uptake indicating a solid tumour; maximal metabolic activity lies within the inferior parts of the tumour mass (PNG 248 kb)
ESM Fig. 3
Case 3. Simultaneously acquired data include structural anatomical data T1-weighted (top row) and FET-PET data (bottom row) (PNG 280 kb)
ESM Fig. 4
Case 4. Simultaneously acquired data include structural anatomical data T1-weighted (top row) and FET-PET data (bottom row). PET data indicate a slightly increased tracer uptake at the inferior margin of the resection area without reaching threshold values indicating a tumour recurrence (PNG 191 kb)
Rights and permissions
About this article
Cite this article
Neuner, I., Kaffanke, J.B., Langen, KJ. et al. Multimodal imaging utilising integrated MR-PET for human brain tumour assessment. Eur Radiol 22, 2568–2580 (2012). https://doi.org/10.1007/s00330-012-2543-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2543-x