Abstract
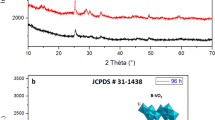
In this paper, 1D single-crystalline MnO2 nanowires have been successfully synthesized by hydrothermal method using KMnO4 and (NH4)2S2O8 as raw materials. X-ray diffraction patterns and high-resolution TEM images reveal pure tetragonal MnO2 phase with diameters of 15–20 nm. Photoluminescence studies exhibited a strong ultraviolet (UV) emission band at 380 nm, blue emission at 452 nm and an extra weak defect-related green emission at 542 nm. UV–visible spectrophotometery was used to determine the absorption behavior of nanostructured MnO2 and a direct optical band gap of 2.5 eV was acquired by Davis–Mott model. The magnetic properties of the products have been evaluated using vibrating sample magnetometer, which showed that MnO2 nanowires exhibited a superparamagnetic behavior at room temperature. The magnetization versus temperature curve of the as-obtained MnO2 nanowires shows that antiferromagnetic transition temperature is 99 K.




Similar content being viewed by others

References
Y. Li, J. Wang, Y. Zhang, M.N. Banis, J. Liu, D. Geng, X. Sun, J. Colloid Interface Sci. 369, 123–128 (2012)
G. Zou, H. Li, Y. Zhang, K. Xiong, Y. Qian, Nanotechnology 17, 313 (2006)
S. Jana, S. Pande, A.K. Sinha, S. Sarkar, M. Pradhan, M. Basu, T. Pal, J. Phys. Chem. C 113, 1386–1392 (2009)
S.C. Pang, S.F. Chin, C.Y. Ling, J. Nano Mater. 2, 607870 (2012)
Z. Pan, Li Xinyong, Z. Qidong, Li Shaomin, Nanoscale Res. Lett. 6, 323 (2011)
A.M. Toufiq, F.P. Wang, Q.U.A. Javed, J. Nanosci. Nanotechnol. 13, 2948 (2013)
B.A. Pinaud, Z. Chen, D.N. Abram, T.F. Jaramillo, J. Phys. Chem. C 115(23), 11830–11838 (2011)
J.E. Greedan, N.P. Raju, A.S. Wills, C. Morin, S.M. Shaw, J.N. Reimers, Chem. Mater. 10(10), 3058–3067 (1998)
H.J. Kim, J.B. Lee, Y.M. Kim, M.H. Jung, Z. Jaglicic, P. Umek, J. Dolinsek, Nanoscale Res. Lett. 2(2), 81–86 (2007)
S. Jana, S. Basu, S. Pande, S.K. Ghosh, T. Pal, J. Phys. Chem. C 111, 16272–16277 (2007)
C.M. Julien, M. Massot, C. Poinsignon, Spectrochimica Acta, Part A 60, 689–700 (2004)
F. Cheng, J. Zhao, W. Song, C. Li, H. Ma, J. Chen, P. Shen, Inorg. Chem. 45, 2038–2044 (2006)
Y. Yang, L. Xiao, Y. Zhao, F. Wang, Int. J. Electrochem. Sci. 3, 67–74 (2008)
X. Wang, Y. Li, J. Am. Chem. Soc. 124, 2880–2881 (2002)
X. Wang, Y. Li, Chem. Eur. J. 9, 300–306 (2003)
J.B. Yang, X.D. Zhou, W.J. James, S.K. Malik, C.S. Wang, Appl. Phys. Lett. 85, 3160–3162 (2004)
B. Li, G. Rong, Y. Xie, L. Huang, C. Feng, Inorg. Chem. 45, 6404–6410 (2006)
Y. Chen, C. Liu, F. Li, H.M. Cheng, J. Alloy Compd. 397, 282–285 (2005)
G.H. Yue, P.X. Yan, D. Yan, D.M. Qu, X.Y. Fan, M.X. Wang, H.T. Shang, J. Cryst. Growth 294, 385–388 (2006)
J.T. Sampanthar, J. Dou, G.G. Joo, E. Widjaja, L.Q.H. Eunice, Nanotechnology 18, 025601 (2007)
H. Wang, Z. Lu, D. Qian, Y. Li, W. Zhang, Nanotechnology 18, 115616 (2007)
X. Chen, X. Li, Y. Jiang, C. Shi, X. Li, Solid State Commun. 136, 94–96 (2005)
J. Luo, H.T. Zhu, H.M. Fan, J.K. Liang, H.L. Shi, G.H. Rao, Z.X. Shen, J. Phys. Chem. C 112, 12594–12598 (2008)
J.G. Zhao, J.Z. Yin, S.G. Yang, Mater. Res. Bull. 47, 896–900 (2012)
M. Zhou, X. Zhang, J. Wei, S. Zhao, L. Wang, B. Feng, J. Phys. Chem. C 115, 1398–1402 (2010)
Y.H. Pai, C.T. Tsai, Int. J. Hydrogen Energy 38, 4342–4350 (2013)
L. Song, S. Zhang, X. Wu, Q. Wei, Chem. Eng. J. 187, 385–390 (2012)
R. Kannan, K. Karunakaran, S. Vasanthkumar, Appl. Nanosci. 1, 197–203 (2011)
S. Li, Z. Ma, L. Wang, J. Liu, Sci. China, Ser. B: Chem. 51(2), 179–185 (2008)
P. Asogwa, J. Optoelectron. Biomed. Mater. 2, 109–117 (2010)
K.A.M. Ahmed, H. Peng, K. Wu, K. Huang, Chem. Eng. J. 172, 531–539 (2011)
A.M. Toufiq, F.P. Wang, Q.U.A. Javed, Y. Li, Nanotechnology 24, 415703 (2013)
N.A. Frey, S. Peng, K. Cheng, S. Sun, Chem. Soc. Rev. 38(9), 2532–2542 (2009)
L. Ying, L. Yongfeng, W. Tianxing, J. Nanomater. 1-6 (2012)
S. Hao-Ling, S. Hongtao, Z. Fei, Q. Limin, G. Song, Chem. Commun. 34, 4339–4341 (2005)
X.M. Liu, S.Y. Fu, C. Huang, J. Powder Technol. 154(2), 120–124 (2005)
H.T. Zhu, J. Luo, H.X. Yang, J.K. Liang, G.H. Rao, J.B. Li, Z.M. Du, J. Phys. Chem. C 112, 17089–17094 (2008)
J. Luo, H.T. Zhu, F. Zhang, J.K. Liang, G.H. Rao, J.B. Li, Z.M. Du, J. Appl. Phys. 105, 093925 (2009)
J. Luo, H.T. Zhu, J.K. Liang, G.H. Rao, J.B. Li, Z.M. Du, J. Phys. Chem. C 114, 8782–8786 (2010)
Acknowledgments
We appreciate the financial supports of National Key Scientific Instruments and Equipment Development Special Fund (2011YQ14014506 and 2011YQ14014507), the Oriented Award Foundation for Science and Technological Innovation, Inner Mongolia Autonomous Region, China (2012), University of Science and Technology Beijing (fundamental development fund and Chinese government scholarship program) and the Fundamental Research Funds for the Central Universities: FRF-BR-09-007A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toufiq, A.M., Wang, F., Javed, Qua. et al. Hydrothermal synthesis of MnO2 nanowires: structural characterizations, optical and magnetic properties. Appl. Phys. A 116, 1127–1132 (2014). https://doi.org/10.1007/s00339-013-8195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-8195-0