Abstract
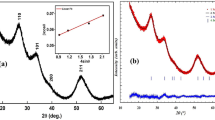
A facile and template-free wet electrochemical technique was used to deposit SnS on tin substrate. Longer time (>40 min) is required for the formation of the chalcogenide thin films and the potential must be carefully controlled to come out with a rough chemical identification of sulfide deposited at low potential scan. The deposition potential is selected from the cyclic voltammetry to preclude the oxidation of SnS to SnS2. The SnS films are uniform and well adhered to the substrate. They were characterized by X-ray diffraction, UV–Vis spectroscopy and electrochemical impedance spectroscopy (EIS). SnS crystallizes in an orthorhombic symmetry (SG: Pnma) and a crystallite size of 42 nm was obtained. The Mott–Schottky plot exhibited a linear behavior with a negative slope, characteristic of p-type conductivity. Holes density of 9.75 × 1020 cm−3, a flat band potential of 0.56 V SCE and a depletion width of ~38 nm were determined. The valence band was located at (−5.41 eV/0.66 V) and derives mainly from S 2−: 3p while the conduction band (3.8 eV/−0.95 V) was primarily made up of Sn2+: 5p orbital. The EIS spectra measured over the frequency range (3 × 10−3–105 Hz) revealed mainly a bulk contribution. On application, rhodamine B was successfully oxidized on SnS films, 38% of the initial concentration (10 mg L−1) disappeared after 4 h of exposure to solar light (90 mW cm−2).











Similar content being viewed by others
Notes
Determined from the dielectric measurement at room temperature on sintered pellet.
References
V.R.M. Reddy, S. Gedi, C. Park, R.W. Miles, K.T.R. Reddy, Development of sulphurized SnS thin film solar cells. Current Appl. Phys. 15, 588–598 (2015)
J. Xu, Y. Yang, Z. Xie, Fabrications of SnS thin films and SnS-based heterojunctions on flexible polyimide substrates. J. Mater. Sci. Mater. Electron. 25, 3028–3033 (2014)
B. Ghosh, M. Das, P. Banerjee, S. Das, Fabrication and optical properties of SnS thin films by SILAR method. Appli Surf Sci 254, 6436–6440 (2008)
S. Gedi, V.R.M. Reddy, C. Park, J. Chan-Wook, K.T.R. Reddy, Comprehensive optical studies on SnS layers synthesized by chemical bath deposition. Optical Mater 42, 468–475 (2015)
M. Mnaria, N. Kamoun, J. Bonnet, M. Dachraoui, Chemical bath deposition of tin sulphide thin films in acid solution. C. R. Chimie 12, 824–827 (2009)
W. Cai, J. Hu, Y. Zhao, H. Yang, J. Wang, W. Xiang, Synthesis and characterization of nanoplate-based SnS microflowers via a simple solvothermal process with biomolecule assistance. Adv. Powder Technol. 23, 850–854 (2012)
J. Chao, Z. Xie, X. Duan, Y. Dong, Z. Wang, J. Xu, B. Liang, B. Shan, J. Ye, D. Chen, G. Shen, Visible-light-driven photocatalytic and photoelectrochemical properties of porous SnS x (x = 1, 2) architectures. Cryst. Eng. Comm. 14, 3163 (2012)
M. Du, X. Yin, H. Gong, Effects of triethanolamine on the morphology and phase of chemically deposited tin sulfide. Mater. Lett. 152, 40–44 (2015)
B. Ghosh, M. Das, P. Banerjee, S. Das, Fabrication of vacuum-evaporated SnS/CdS heterojunction for PV applications. Solar Energy Mater. Solar Cells 92, 1099–1104 (2008)
X.L. Gou, J. Chen, P.W. Shen, Gou synthesis, characterization and application of SnS x (x = 1, 2) nanoparticles. Mater. Chem. Phys. 93, 557–566 (2005)
C. Cifuentes, M. Botero, E. Romero, C. Calder, G. Gordillo, Optical and structural studies on SnS films grown by co-evaporation. Braz. J Phys. 36, 1046–1049 (2006)
S. Omeiri, B. Hadjarab, M. Trari, Photoelectrochemical properties of anodic silver sulphide thinfilms. Thin Solid Films 519, 4277–4281 (2011)
A. Gómez, H. Martínez, M.C. Rodríguez, D. Avellaneda, P.G. Reyes, O. Flores, A Study of the Structural, Optical and electrical properties of SnS thin films modified by plasma. J. Mater. Sci. Eng. B. 6, 352–358 (2013)
O.E. Ogah, G. Zoppi, I. Forbes, R.W. Miles, Thin films of tin sulphide for use in thin film solar cell devices. Thin Solid Films 517, 2485–2488 (2009)
A. Akkari, C. Guasch, N.K. Turki, Chemically deposited tin sulphide. J. Alloys Compounds 490, 180–183 (2010)
K.S. Kumar, C. Manoharan, S. Dhanapandian, A.G. Manohari, T. Mahalingam, Effect of indium incorporation on properties of SnS thin films prepared by spray pyrolysis. Optik 125, 3996–4000 (2014)
S. Kaizra, Y. Louafi, B. Bellal, M. Trari, G. Rekhila, Electrochemical growth of tin (II) oxide films: application in photocatalytic degradation of methylene blue. Mater. Sci. Semicond. Process 30, 554–560 (2015)
L.M. Peter, The electrocrystallisation of cadmium sulphide films on cadmium. Electrochim. Acta 23, 165–174 (1978)
J. Chao, Z. Wang, X. Xu, Q. Xiang, W. Song, G. Chen, J. Hu, D. Chen, Tin sulfide nanoribbons as high performance photoelectrochemical cells, flexible photodetectors and visible-light-driven photocatalysts. RSC Adv. 3, 2746–2753 (2013)
R. Brahimi, Y. Bessekhouad, A. Bouguelia, M. Trari, CuAlO2/TiO2 heterojunction applied to visible light H2production. J. Photochem. Photobiol. A 186, 242–247 (2007)
S.H. Chaki, M.D. Chaudhary, M.P. Deshpande, Synthesis and characterization of different morphological SnS nanomaterials. Adv. Nat. Sci. Nanosci. Nanotechnol. 5, 045010–0450119 (2014)
W.M. Haynes, Handbook of Chemistry and Physics, 95th edn. (CRC Press, Boca Raton, 2014)
M. Amaraa, H. Kerdjoudj, A. Bouguelia, M. Trari, A combination between membrane selectivity and photoelectrochemistry to the separation of copper, zinc and nickel in aqueous solutions. J. Membr. Sci. 312, 125–131 (2008)
S. Boumaza, A. Boudjemaa, S. Omeiri, R. Bouarab, A. Bouguelia, M. Trari, Physical and photoelectrochemical characterizations of hematite α-Fe2O3: application to photocatalyticoxygen evolution. Sol. Energy 84, 715–721 (2010)
Y.D. Tembhurkar, n–CuInS2/polysulfide photoelectrochemical solar cells prepared by spray pyrolysis. Bull. Mater. Sci. 20, 1011–1014 (1997)
B. Show, N. Mukherjee, A. Mondal, Electrochemically synthesized microcrystalline tin sulphide thin films: high dielectric stability with lower relaxation time and efficient photochemical and photoelectrochemical properties. RSC Adv 4, 58740–58751 (2014)
Acknowledgements
This work was supported by the Faculty of Chemistry (U.S.T.H.B., Algiers). The authors would like to thank Pr. A. Djadoun for the XRD patterns.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kabouche, S., Louafi, Y., Bellal, B. et al. Electrochemical growth of SnS thin film: application to the photocatalytic degradation of rhodamine B under visible light. Appl. Phys. A 123, 545 (2017). https://doi.org/10.1007/s00339-017-1155-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1155-3