Abstract
Absorption and TDLA spectroscopies find their applications in a lot of fields of research. The purpose of this article is to show how these methods can bring significant advances in chemical research projects. “H2 massive production” using nuclear heat together with a thermochemical cycle is an important way to massively produce hydrogen, a potential energy vector. The sulfur–iodine cycle and the hybrid copper-chloride thermochemical cycles are some good candidates for water splitting.
In the case of the sulfur-iodine thermochemical cycle, the overall efficiency of the process essentially depends on the efficiency of HI section. Using optical techniques, such as a FTIR spectrometer for H2O and HI concentrations determination, and a TDL spectrometer for I2 measurements, it enables to get very significant results that will be useful to build a new thermodynamic model of the HI separation. This nonintrusive method has avoided any vapor change and prevented tedious experiments in harsh environments.
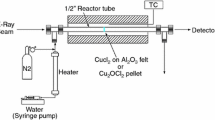
The same methodology is now applied for the study of the hydrolysis reaction of the thermochemical hybrid copper-chloride cycle. The study of this reaction is very important to assess the viability of this cycle because this reaction is not thermodynamically favored and it only occurs if a large excess of water is used. To better understand the influence of various parameters, such as water stoichiometry, temperature, reaction duration, an experimental setup has been designed and realized. The experimental setup uses two spectrometers to study the speciation of the gaseous phase and optimize the kinetics of the hydrolysis reaction. Concentrations of HCl and H2O are obtained by fitting experimental FTIR spectra with calculated spectra. Parasitic reactions can appear leading to formation of Cl2, measured by UV–Visible spectrophotometry.
The high temperature reaction at around 530°C is the only reaction of this copper-chloride cycle which is thermodynamically favored. A better understanding of its kinetics and the influence of the experimental parameters on this kinetics are needed. For this purpose, an absorption spectrometer able to measure oxygen is under study. This instrument is based on a high-finesse cavity and a DFB diode in order to access an oxygen absorption line spectra, near 1.3 μm. Preliminary results of this work will be presented.
Similar content being viewed by others
References
J.M. Hartmann, L.H. Coudert, D. Doizi, V. Dauvois, J.L. Roujou, V. Lorin, B. Larousse, P. Fauvet, P. Carles, Int. J. Hydrogen Energy 34, 162 (2009)
D. Doizi, V. Dauvois, J.L. Roujou, V. Delanne, P. Fauvet, B. Larousse, O. Hercher, P. Carles, C. Moulin, J.M. Hartmann, Int. J. Hydrogen Energy 32, 1183 (2007)
M.S. Ferrandon, M.A. Lewis, D.F. Tatterson, A. Gross, D. Doizi, L. Croizé, V. Dauvois, J.L. Roujou, Y. Zanella, P. Canes. Int. J. Hydrogen Energy, Ms. Ref. No. HE-D-09-01482R1 (in press)
M.A. Lewis, J.G. Masin, Int. J. Hydrogen Energy 34, 4125 (2009)
D. Doizi, V. Dauvois, J.L. Roujou, V. Lorin, P. Fauvet, B. Larousse, P. Carles, J.M. Hartmann, Int. J. Hydrogen Energy 34, 4275 (2009)
B. Larousse, P. Lovera, J.M. Borgard, G. Roehrich, N. Mokrani, C. Maillault, D. Doizi, V. Dauvois, J.L. Roujou, V. Lorin, P. Fauvet, P. Carles, J.M. Hartmann, Int. J. Hydrogen Energy 34, 258 (2009)
L. Rothman, D. Jacquemart, A. Barbe, D.C. Benner, L.R. Brown, M.R. Carleer, C. Chackerian, Jr., K. Chance, V. Dana, V.M. Devi, J.-M. Flaud, R.R. Gamache, A. Goldman, J.-M. Hartmann, K.W. Jucks, A.G. Maki, J.-Y. Mandin, S. Massie, A. Perrin, C.P. Rinsland, M.A.H. Smith, R.A. Toth, J. Vander Auwera, P. Varanasi, JQSRT 96, 139 (2005)
R.S. Mulliken, Phys. Rev. 32, 880 (1928)
D.A.G. Deacon, A. O’Keefe, Rev. Sci. Instrum. 59, 2544 (1988)
J. Morville, S. Kassi, M. Chenevier, D. Romanini, Appl. Phys. B 80, 1027 (2005)
D.W. Ferguson, K.N. Rao, M.E. Mickelson, L.E. Larson, J. Mol. Spectrosc. 160, 315 (1993)
C. Neumann, Diplomaufgabe. RWTH Aachen, Aachen (1987)
H. Engels, K.F. Knoche, Int. J. Hydrogen Energy 11, 703 (1986)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Croizé, L., Doizi, D., Larousse, B. et al. Interest of absorption spectroscopy for the control of industrial processes. Application to H2 massive production. Appl. Phys. B 100, 409–415 (2010). https://doi.org/10.1007/s00340-009-3853-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-009-3853-9