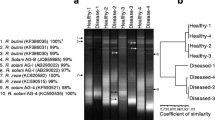
Abstract
The effects of intercropping with maize and Rhizobium inoculation on the yield of faba bean and rhizosphere bacterial diversity were analyzed by terminal restriction fragment length polymorphism, amplified 16S rDNA restriction analysis (ARDRA), and 16S rDNA sequencing. The results showed that intercropping but not Rhizobium inoculation significantly increased the faba bean yield. Probably the relatively high level of native rhizobia in soil annulled the effect of rhizobia inoculation. ARDRA results showed that intercropping did not affect bacterial diversity whereas Rhizobium inoculation decreased bacterial diversity. The canonical correspondence analysis showed that the composition of bacterial community was changed apparently by intercropping, and there was a positive correlation (P = 0.724) between faba bean yields and intercropping and an apparent correlation (P = 0.648) between intercropping and total N. The available content of K and P had a lower effect on the bacterial community composition than did the total N content, Rhizobium inoculation, and microbial biomass C. Rhizobium inoculation negatively correlated with microbial biomass C (P = −0.827). These results revealed a complex interaction among the intercropped crops, inoculation with rhizobia, and indigenous bacteria and implied that the increase of faba bean production in intercropping might be related to the modification of rhizosphere bacterial community.








Similar content being viewed by others
References
Alkorta I, Amezaga I, Albizu I, Aizpurua A, Onaindia M, Buchner V, Garbisu C (2003) Molecular microbial biodiversity assessment: a biological indicator of soil health. Rev Environ Health 18:131–151
Arriagada CA, Herrera MA, Ocampo JA (2007) Beneficial effect of saprobe and arbuscular mycorrhizal fungi on growth of Eucalyptus globulus co-cultured with Glycine max in soil contaminated with heavy metals. J Environ Manage 84:93–99. doi:10.1016/j.jenvman.2006.05.005
Balota EL, Colozzi-Filho A, Andrade DS, Dick RP (2003) Microbial biomass in soils under different tillage and crop rotation systems. Biol Fertil Soils 38:15–20. doi:10.1007/s00374-003-0590-9
Bandyopadhyay SK, De R (1986) N relationship in a legume non-legume association grown in an intercropping system. Fert Res 10:73–82. doi:10.1007/BF01073906
Boucher DH, Espinosa J (1982) Cropping system and growth and nodulation responses of beans to nitrogen in Tabasco, Mexico. Trop Agr 59:279–282
Bowman JP, McCuaig RD (2003) Biodiversity, community structural shift, and biogeography of prokaryotes within Antarctic continental shelf sediment. Appl Environ Microbiol 69:2463–2483. doi:10.1128/AEM.69.5.2463-2483.2003
Davelos AL, Xiao K, Samac DA, Martin AP, Kinkel LL (2004) Spatial variation in Streptomyces genetic composition and diversity in a prairie soil. Microb Ecol 48:601–612. doi:10.1007/s00248-004-0031-9
De Ridder-Duine AS, Kowalchuk GA, Klein Gunnewiek PJA, Smant W, Van Een JA, de Boer W (2005) Rhizosphere bacterial community composition in natural stands of Carex arenaria (sand sedge) is determined by bulk soil community composition. Soil Biol Biochem 37:349–357. doi:10.1016/j.soilbio.2004.08.005
Di Cello FD, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C (1997) Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol 63:4485–4493. doi:0099-2240/97/$04.0010
Dunbar J, Ticknor LO, Kuske CR (2001) Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl Environ Microbiol 67:190–197. doi:10.1128/AEM.67.1.190-197.2001
Franchini JC, Crispino CC, Souza RA, Torres E, Hungaria M (2007) Microbiological parameters as indicators of soil quality under various soil management and crop rotation systems in southern Brazil. Soil Till Res 92:18–29. doi:10.1016/j.still.2005.12.010
Good IL (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264. doi:10.2307/2333344
Grayston SJ, Wang SQ, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378. doi:10.1016/S0038-0717(97)00124-7
Hanway JJ, Heidal H (1952) Soil analysis methods used in Iowa State College, 57th edn. Soil Testing Laboratory, Iowa Agron, pp 1–31. doi:10.1016/S0038-0717(97)00124-7
Hao YR, Lao XR, Sun WH, Peng SL (2003) Interaction of roots and rhizosphere in the wheat/maize intercropping system. J Ecol Rural Environ 19:18–22
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain leguminous in the tropics, with an emphasis on Brazil. Field Cr Res 65:151–164. doi:10.1016/S0378-4290(99)00084-2
Junier P, Junier T, Witzel KP, Caru M (2009) Composition of diazotrophic bacterial assemblages in bean-planted soil compared to unplanted soil. Eur J Soil Sci 45:153–162. doi:10.1016/j.ejsobi.2008.10.002
Khan MS, Zaidi A, Amil M (1997) Associative effect of Bradyrhizobium sp. (Vigna) and phosphate solubilizing bacteria on mungbean [Vigna radiata (L.) Wilczek]. Biojounal 9:101–106
Kjeldahl J (1883) Neue methode zur bestimmung des stickstoffs in organischen körpern. Z Anal Chem 22:366–382. doi:10.1007/BF01338151
Lerner A, Herschkovitz Y, Baudoin E, Nazaret S, Loccoz YM, Okon Y, Edouard J (2006) Effect of Azospirillum brasilense inoculation on rhizobacterial communities analyzed by denaturing gradient gel electrophoresis and automated ribosomal intergenic spacer analysis. Soil Biol Biochem 38:1212–1218. doi:10.1016/j.soilbio.2005.10.007
Li L, Li SM, Sun JH, Zhou LL, Bao XG, Zhang HG, Zhang FS (2007) Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc Natl Acad Sci USA 104:11192–11196. doi:10.1073/pnas.0704591104
Li HG, Shen JB, Zhang FS, Marschner P, Cawthray G, Rengel Z (2010) Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol Fertil Soils 46:79–91
Nielsen MN, Winding A (2002) Microorganisms as indicators of soil health. Ministry of the Environment, National Environmental Research Institute, Denmark
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939
Park S, Ku YK, Seo MJ, Kim DY, Yeon JE, Jeong SC, Yoon WK, Kim HM (2006) The characterization of bacterial community structure in the rhizosphere of watermelon (Citrullus vulgaris Schard.) using culture-based approaches and terminal fragment length polymorphism (T-RFLP). Appl Soil Ecol 33:79–86. doi:10.1016/j.apsoil.2005.08.006
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144. doi:10.1016/0022-5193(66)90013-0
Ravenschlag K, Sahm K, Pernthaler J, Amann R (1999) High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol 65:3982–3989
Rooney DC, Clipson N (2008) Impact of sheep urine deposition and plant species on ammonia-oxidizing bacteria in upland grassland soil. Can J Microbiol 54:791–796. doi:10.1139/W08-065
Saini VK, Bhandari SC, Tarafdar JC (2004) Comparison of crop yield, soil microbial C, N and P, N-fixation, nodulation and mycorrhizal infection in inoculated and non-inoculated sorghum and chickpea crops. Field Cr Res 89:39–47. doi:10.1016/j.fcr.2004.01.013
Sandnes A, Eldhuset TD, Wollebæk G (2005) Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biol Biochem 37:259–269. doi:10.1016/j.soilbio.2004.07.036
Singh BK, Munro S, Potts JM, Millard P (2007) Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl Soil Ecol 36:147–155. doi:10.1016/j.apsoil.2007.01.004
Smith CJ, Danilowicz BS, Clear AK, Costello FJ, Wilson B, Meijer WG (2005) T-Align, a web-based tool for comparison of multiple terminal restriction fragment length polymorphism profiles. FEMS Microbiol Ecol 54:375–380. doi:10.1016/j.femsec.2005.05.002
Sneath PHA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco. doi:10.1007/0-387-28021-9_6
Song YN, Zhang FS, Marschner P, Fan FL, Gao HM, Bao XG, Sun JH, Li L (2007) Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol Fert Soils 43:565–574. doi:10.1007/s00374-006-0139-9
Stolp H (1988) Microbial ecology: habitats, activities. Cambridge University Press, Cambridge, pp 221–223. doi:10.1016/0769-2609(88)90090-7
Thies JE, Singleton PW, Bohlool BB (1991) Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol 57:19–28. doi:0099-2240/91/010019-10.00/0
Thompson JR, Marcelino LA, Polz MF (2002) Heteroduplexes in mixed-template amplification: formation, consequence and elimination by ‘reconditioning PCR’. Nucleic Acids Res 30:2083–2088. doi:10.1093/nar/30.9.2083
Tobita S, Ito O, Matsunaga R, Rao TP, Rego TJ, Johansen C, Yoneyama T (1994) Field evaluation of nitrogen fixation and use of nitrogen fertilizer by sorghum/pigeonpea intercropping on an Alfisol in the Indian semi-arid tropics. Biol Fert Soils 17:241–248. doi:10.1007/BF00383976
Tomasi N, Weisskopf L, Renella G, Landi L, Pinton R, Varanini Z, Nannipieri P, Torrent J, Martinoia E, Cesco S (2008) Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem 40:1971–1974. doi:10.1016/j.soilbio.2008.02.017
Torsvik V, Goksoyr J, Daae FL (1990) High diversity in DNA of soil bacteria. Appl Environ Microbiol 56:782–787. doi:0099-2240/90/030776-06.00/0
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. doi:10.1016/0038-0717(87)90052-6
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell, Oxford. doi:10.2307/2402718
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38. doi:10.1097/00010694-193401000-00003
Xiao YB, Li L, Zhang FS (2004) Effect of root contact on interspecific competition and N transfer between wheat and faba bean using direct and indirect 15N techniques. Plant Soil 262:45–54. doi:10.1023/B:PLSO.0000037019.34719.0d
Zhou JZ, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322. doi:0099-2240/96/$04.00±0
Acknowledgments
This study was funded by the National Basic Research (973) program of China (project no. 2006CB100206). We thank Mr. Jian Hao Sun and Professor Xing Guo Bao for technical assistance. The field experiments were performed with the assistance from the Institute of Soil Science and Fertilizers, Gansu Academy of Agricultural Sciences. Ms. Hui Min Gao helped us in the sample collection. We thank Professor Kate Scow for her valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, N.N., Sun, Y.M., Li, L. et al. Effects of intercropping and Rhizobium inoculation on yield and rhizosphere bacterial community of faba bean (Vicia faba L.). Biol Fertil Soils 46, 625–639 (2010). https://doi.org/10.1007/s00374-010-0469-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-010-0469-5