Abstract
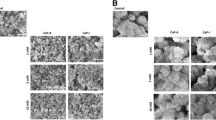
We studied the possible use of silicon carbide (SiC) as a ceramic coating material of titanium-based total hip replacement (THR) implants. The idea is to prevent wear debris formation from the soft titanium surface. SiC is a hard and tightly bonding ceramic surface material, and because of these physical properties it is not easily degradable, as is the case with hydroxyapatite. Our previous in vivo and in vitro studies have indicated that SiC and hydroxyapatite are equally biocompatible regarding particle size for phagocytosis. The present cytotoxicity test using JCRB0603 cells showed that 5 μm SiC particles inhibited colony outgrowth by one-third (67% + 10% vs control), while SiC-coated pins did not cause any inhibition and acted similarly to uncoated titanium pins. The results support the hypothesis that SiC is a promising ceramic THR implant coating material.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 23 May 1997
Rights and permissions
About this article
Cite this article
Santavirta, S., Takagi, M., Nordsletten, L. et al. Biocompatibility of silicon carbide in colony formation test in vitro . Arch Orth Traum Surg 118, 89–91 (1998). https://doi.org/10.1007/s004020050319
Issue Date:
DOI: https://doi.org/10.1007/s004020050319