Abstract
The etiology of multiple sclerosis has not yet been fully described. A potential link between the recombinant hepatitis B vaccine and an increased risk of onset or exacerbation of multiple sclerosis emerged in the mid-1990s, leading to several spontaneous reports and studies investigating this association. We conducted a critical systematic review aimed at assessing whether hepatitis B vaccination increases the risk of onset or relapse of multiple sclerosis and other central nervous system demyelinating diseases. MEDLINE and EMBASE were used as data sources, and the search covered the period between 1981 and 2011. Twelve references met the inclusion criteria. No significant increased risk of onset or relapse of the diseases considered was associated with hepatitis B vaccination, except in one study. Most studies included in this review displayed methodological limitations and heterogeneity among them, which rendered it impossible to draw robust conclusions about the safety of hepatitis B vaccination in healthy subjects and patients with multiple sclerosis. Therefore, on the basis of current data there is no need to modify the vaccination recommendations; however, there is a need to improve the quality of observational studies with emphasis on certain considerations that are discussed in this review.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder of the central nervous system (CNS) [1]. It is estimated that about 2–2.5 million people worldwide suffer from MS [2]. Twice as many women as men are affected, and the age of onset is usually between 20 and 40 years [1]. MS is generally considered to be an autoimmune disease directed against CNS myelin or oligodendrocytes, with a multifactorial pathogenesis that appears to involve both genetic and environmental factors [2]. It has been suggested that immune responses generated by infections or vaccinations may cause, trigger or exacerbate CNS autoimmunity in susceptible individuals [3, 4]. In this regard, the implementation of universal hepatitis B (HB) vaccination has been controversial [5].
Hepatitis B is an important public health problem worldwide; about one-third of the world’s population has been infected with the HB virus. Recombinant HB vaccines became available in the mid-1980s, and to date, several hundred million doses have been administered, with successful results as regards safety and efficacy [6]. Nevertheless, several reports of symptoms of CNS demyelination shortly after vaccination have raised concerns about whether the HB vaccine may lead to new cases or relapses of MS (or other CNS demyelinating diseases) [7].
The limited knowledge of the etiology of MS, combined with the low incidence of cases that have been associated with vaccination, make it difficult to study this association. The only evidence available relies on case reports, molecular studies [8] or hypothesis [9, 10] concerning the possible biological plausibility [11], and epidemiological studies. Although the results of most epidemiological studies are consistent with a null association, some controversy remains. Hence, we carried out a critical systematic review to provide an updated report on the safety of the vaccine in both healthy individuals and patients with MS.
Methods
Data sources and selection criteria
A search of the scientific MEDLINE and EMBASE databases was performed for the period between January 1981 and December 2011. Other search engines, such as Google Scholar, were also used. The lower limit of time was established as 5 years before the year in which the first genetically engineered vaccine against HB was commercialized, to enable the location of any clinical trials.
The following search terms were used: “hepatitis B” AND (vaccine OR vaccination OR immunization) AND (autoimmunity OR “autoimmune disease” OR “multiple sclerosis” OR demyelination). As an additional measure to avoid possible oversights, the references cited in retrieved articles were used to locate other articles. The selection criteria were as follows: (i) the studies had to be original; and (ii) the stated objective of the studies had to be to assess the relationship between recombinant HB vaccine and the onset or relapse of MS, or a first CNS demyelinating event (as this may be consistent with MS, although the criteria for definitive diagnosis have not yet been fulfilled [1]). Molecular studies and studies based on case reports and case series were excluded.
Data extraction
For each study, a table including the following parameters was drawn up: author, year of publication, country, age, main outcome variable, study design and number of subjects in the study and comparison groups, definition of each group, sources of the outcome and exposure data, and time window considered in the primary hypothesis (Table 1).
Results corresponding to the main analyses involved in the studies are summarized in Table 2 using the following data: author; descriptive data, with positive and negative subjects in each group; and relative risk (RR) plus confidence interval (CI), or significance. Positive subjects refer to subjects who developed the disease in time-series and cohort studies, or those who were vaccinated against HB in case–control studies. Negative subjects refer to the opposite status. When data in the contingency table were incomplete, they were calculated from the data presented in the study, and when an effect measure was not provided, crude measures were obtained by use of the Epidat 4.0 software package.
Results
Selection of papers
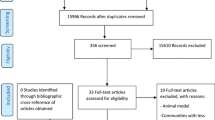
A total of 216 and 190 studies were identified via MEDLINE and EMBASE, respectively. After reviewing the chosen literature, a total of 13 studies were selected [12–24]. References included in all articles were scanned, but no relevant citations were added to the collection. Of these, the study performed by Mikaeloff et al. [21] was not included in this review, to avoid duplication, as these authors published a subsequent paper conducted in the same population and with a broader definition of the outcome. The selection process is shown in the flow diagram (see Fig. 1).
Regarding the geographical distribution, five studies were conducted in France [14, 16, 17, 22, 24], one of which also included Spain and Switzerland [16]. Three studies were performed in the USA [12, 15, 18], two in Canada [13, 23], one in the UK [19] and one in Turkey [20] (Table 1).
Methods used in the selected studies
The study design was specified in 9 of the 12 papers. Six claimed to be case–control studies [14, 15, 17–19, 24], and three of these were nested in one [19, 24] or two cohorts [15]; two studies claimed to be cohort studies [12, 22], and one [16] was stated to be a case-crossover study. In the case–control studies, sample size ranged from 121 to 14,362 individuals in the case group, and from 121 to 7,671 in the control group. In the cohort studies, sample size varied from 11 to 27,229 subjects in the study group, and from 71 to 107,469 in the comparison group. An ecological study [13] estimated 289,651 individuals in the study group and 288,657 in the comparison group, and the case-crossover study [16] included 643 patients (Table 1).
Eight studies [14–20, 23] were conducted in adults, three [13, 22, 24] were carried out in children, and one [12] included all ages, although 65 % of person-years corresponded to children (Table 1).
Assessment of exposure to HB vaccine
Nine studies requested information from participants about exposure [14–18, 20, 22–24]. In addition, subjects were previously contacted in seven studies by means of a letter/postal questionnaire [14–17, 22, 24] and/or by telephone [18, 22, 24], with participation rates that ranged from 71.9 to 95.1 % in study groups and from 50.4 to 87.8 % in comparison groups. Among the methods for obtaining information were postal questionnaires [14, 15, 24], telephone interviews [16–18, 22] and personally administered questionnaires [23]. Only one study [20] failed to mention the data collection method. All of these studies, except for that by Ramagopalan et al. [23], used at least one of the following additional methods to confirm the vaccination status: vaccination certificates [14, 16, 17, 20, 22, 24], medical records [15, 18], telephone interviews [14, 24], and confirmation from the physician [16]. Finally, of these studies, three included unconfirmed subjects for the main analysis [14, 17, 18], with confirmation percentages that ranged from 36.7 to 88.9 % of the total participants. The remaining studies included only confirmed subjects, although Ascherio et al. [15] excluded only unconfirmed women who reported having been vaccinated. Other studies used as sources to determine exposure pharmacy and medical claims [12], medical records [19] and the Centre for Disease Control [13] (Table 1).
Criteria used to classify individuals as exposed were explicitly mentioned in the methodology in seven studies [13–16, 18, 19, 22]. In studies that analysed specific time windows, only three [15, 20, 24] considered as unexposed subjects who were not vaccinated between birth and disease onset, whereas the remaining studies did not assess or specify [12, 14, 16, 17, 19, 22] whether unexposed subjects could have been vaccinated prior to the period considered.
Assessment of the outcome
Five studies [13, 15, 18, 19, 23] evaluated the risk of MS onset, of which one [18] also included optic neuritis. In three studies [14, 17, 24], the main outcome was a first episode of CNS demyelination, whatever the course of the disease. Zipp et al. [12] considered any CNS demyelinating disease. Two studies evaluated the relapse rate and degree of disability [20], and the risk of relapse [16] in MS patients, and Mikaeloff et al. [22] evaluated the risk of relapse that determines conversion to MS. Of these studies, four [12, 13, 23, 24] failed to mention or did not apply standardized diagnostic criteria.
As regards the sources of diagnostic information, seven studies [13, 14, 16–19, 24] reviewed medical records, one study [12] requested pharmacy and medical claims, and two studies [15, 23] screened individuals with MS by means of a questionnaire; although of these studies, one [15] also reviewed medical records. Finally, information was reported by a trained neurologist in one study [22] and one study [20] did not mention the data source (Table 1).
Etiological window and assessment of the index date
The time window considered in studies ranged widely, from 2 months to 6 years, or was indefinite (Table 1).
Nine studies determined the outcome and the exposure retrospectively [12, 14–19, 23, 24], which makes it necessary to establish an index date for the onset of the disease to enable proper classification of each exposure. All case–control studies considered the date of the first neurological symptoms as the index date, except for the study by Ramagopalan et al. [23], who did not establish an index date. Of these studies, Ascherio et al. [15] chose the date indicated by the patient or the physician, two studies obtained the date from medical records [17, 19], DeStefano et al. [18] used both methods, and two studies did not mention the source of the index date [14, 24]. Finally, Confavreux et al. [16] used the date of medical visit or hospitalization to establish the index date of the relapse and Zipp et al. [12] did not mention whether they considered an index date.
Statistical analysis
Eight studies adjusted for several covariables [14, 15, 17–19, 22–24]. Four of the studies adjusted for cigarette smoking [15, 18, 19, 24], and two considered infections [15, 22]. Four studies adjusted for birth location [14, 15, 17, 18], three evaluated the residence location [14, 17, 22], and two adjusted for ancestry [15, 18]. A family history of demyelinating diseases or other autoimmune diseases was included in three studies [18, 22, 24]. Other covariables considered for statistical adjustment were age and calendar time at onset, sex, number of children, occupation, educational level, other vaccinations, and clinical characteristics.
Results of the papers
As regards the RR obtained (Table 2), four studies [12, 14, 17, 24] found no significant increase of risk of CNS demyelination within 2 or 6 months, and 3 years or more after vaccination, although Touzé et al. [17] did not reject the possibility of a slight increase of risk.
In the evaluation of the risk of MS onset, Sadovnick and Scheifele [13] reported no significant differences in MS incidence in adolescents 7 years before and 6 years after the HB vaccination campaign, and three studies [15, 18, 23] concluded an absence of an increased risk of MS in adults vaccinated within 2 years or at any time. On the other hand, Hernán et al. [19] reported a significant increase of risk of MS in adults within 3 years before the index date, with a crude OR = 3.1 (95 % CI 1.5–6.3).
As regards relapses, Confavreux et al. [16] concluded a lack of an increased risk of relapse in adults for a 2-month risk period, and Mikaeloff et al. [22] reported that vaccination after a first demyelinating episode in children did not appear to increase the risk of conversion to MS within a mean follow-up of 5.8 ± 2.7 years, although they did not exclude the possibility of a slightly increased risk. Finally, in a 2-year follow-up study of patients after the onset of MS, Özakbas et al. [20] found no clinical differences between patients vaccinated before MS onset and unvaccinated MS patients, and thus stated that the vaccine appears to be safe in both healthy and MS patients (Table 2).
Discussion
Discussion of methods
Three studies omitted to specify the design used: an uncontrolled time-series study [13], a cohort study [20] and a case–control study [23]. Causal inference in time-series studies may be limited due to changes in diagnostic criteria of MS and its recording in medical records over time and among different neurologists. Confavreux et al. [16] conducted a case-crossover study. This may not be the best method of assessing the risk of relapse, because of the potential inaccuracy in allocating each vaccination and also because it is assumed that the risk is constant after each vaccination and therefore, the potential dose–response effect is ignored. Moreover, patients with frequent relapses, in whom there may be a greater risk of exacerbation after vaccination, were excluded from the study [25].
The small sample sizes used by Touzé et al. [14] and Özakbas et al. [20] greatly limit the statistical power of the studies. The relatively low incidence and long latent period of demyelinating diseases may also contribute to a lack of statistical power in two studies with a cohort [12] and a time-series design [13], especially as they involved children, who represent <5 % of MS cases [5]. A low-frequency of exposure to the vaccine may also contribute to difficulties in drawing conclusions in four studies [16–19] (Table 2).
Assessment of exposure to HB vaccine
Among the studies that requested information from participants about exposure, the percentage of participation was generally high, although recruitment through letter/postal questionnaire was slightly more successful than through telephone. The greatest differences were observed in case–control studies, as participation among cases was higher than that of controls. In addition, participation was higher in controls selected from hospitals than from the general population.
Most studies attempted to confirm data of exposure through the vaccination certificate, which is a very valuable record of all or most of the vaccines received in a lifetime. Other sources were self-reports, which may be inaccurate due to recall bias, and medical records, which may present large differences in their completeness. Considering the period in which some studies were conducted, it is possible that both medical records and vaccination certificates were incomplete, as HB vaccination was at first targeted towards high-risk individuals [26], such as healthcare workers, who may have been vaccinated at their place of work [27]. The percentage of exclusion in studies which only included subjects with confirmed vaccinations was low, except in one study [15], which excluded more than half of the individuals who reported having been vaccinated (in order to avoid recall bias) and none of the unconfirmed unexposed subjects leading to a possible differential misclassification bias [28, 29].
In most studies, exposure was not well defined, and subjects were usually considered as exposed if they had received any injection during the time window considered, regardless of the number of doses, so that any dose–response effect was overlooked. Regarding studies that evaluated specific time intervals, most did not consider possible vaccination prior to the time window of study. As the latent period of MS is unknown, unexposed subjects should ideally never have been vaccinated. Another aspect not generally considered was the brand of the vaccines, inasmuch as they present variations in their composition [6, 24]. Moreover, in earlier studies, individuals may have been vaccinated with plasma-derived vaccines [30].
Assessment of the outcome
Several studies evaluated the risk of MS onset, which has the disadvantage of excluding any other patients with CNS demyelinating events. As such events may actually be first attacks of MS, exclusion of these from studies may have led to underestimation of the RR [28, 31]. Regarding the study of relapses, studies are susceptible to potential bias derived from differences in the severity of the disease among subjects, as this determines the likelihood of being vaccinated.
Among the studies that mentioned the diagnostic criteria, there was great variability for each outcome criteria, as well as in the level of diagnostic certainty considered. This, together with the lack of a precise definition of the outcome in some studies, leads to inter-study heterogeneity in diagnosis of the same outcome and also intra-study heterogeneity because of differences in diagnosis and data recording between physicians.
Etiological window and assessment of the index date
It has been suggested that exposure to an environmental factor in early life may be implicated in the pathogenesis of MS, with an interval of more than a decade to the disease onset [32]. Nevertheless, most studies considered short time windows, which are more suitable for evaluating an acute reaction.
Final diagnosis of the disease may come several years after the molecular phase and manifestation of the first symptoms, which makes assessment of the disease onset very difficult. This is important in retrospective studies, since inaccurate estimation leads to wrong classification of exposure, which will be even less accurate if short time windows are considered. This may lead to bias and underestimation of the RR, as the probability of exposure may decrease after manifestation of the first symptoms [26, 30]. Regarding the source of the index date, the main advantage of medical records is that information is prospectively recorded [28], although many of the events described may have occurred before the evaluation date. There may be recall bias in the dates reported by subjects, and the date of visit or hospitalization may be even more imprecise, since symptoms may have appeared some time before patients were medically evaluated.
Statistical analysis
Since the etiology of MS is still unclear, it is difficult to determine which confounding factors should be considered for analysis. Hence, some studies considered potential risk factors of MS described in the literature, such as ancestry, cigarette smoking or infectious mononucleosis. Another possible risk factor of MS, which may also affect the prescription of the HB vaccine, is a family history of autoimmune disease [1, 2]. Study of relapses is complex because of the variability in the course of the disease, which determines the number of relapses and the probability of exposure, so that it is difficult to account for all confounding factors.
Discussion of results
Most studies included in this review reported a lack of association between the exposure to HB vaccine and the onset or relapse of a demyelinating disease, even in adults or children, and within any time window considered. The only authors to obtain a significant OR in the main analysis were Hernán et al. [19], although Mikaeloff et al. [24] found a significant association in a secondary analysis. Nevertheless, the latter should be considered inconclusive because of the small number of exposed subjects and the large number of subgroup analyses performed, which increases the probability of detecting a significant result through pure chance [5, 33]. It is not possible to draw conclusions about the vaccine safety in both healthy and MS patients in the study by Özakbas et al. [20] as participants already had MS before the follow-up period, and the study group was vaccinated prior to the onset of the disease.
The global evidence from the results is difficult to interpret in light of the methodological limitations identified. Therefore, it is not possible to conclude whether there is an increased risk of onset or relapse of MS or other demyelinating diseases. Nonetheless, several previous systematic reviews [34, 35], and review committees such as the US Institute of Medicine [1] and the WHO Global Advisory Committee on Vaccine Safety, have concluded that there is evidence for rejecting such an association [36].
Another source of scientific literature relies on a great number of case reports, case series and spontaneous notifications from epidemiological databases [37–39]. The major constraint to draw any conclusion from these studies is that the total number of cases is not known, because of under-reporting [39], so that it is not possible to calculate any effect measures. Furthermore, the reporting rate of an adverse effect is influenced by various factors; for example, the reporting rate increases when public alarm is generated, as occurred in France when several reports of CNS demyelinating disorders following HB vaccination led the French government to suspend the vaccination program in adolescents in 1998 [40]. This also explains why this country currently has one of the lowest rates of immunization against HB among developed countries [41, 42].
Limitations
As the search was restricted to MEDLINE and EMBASE, it is possible that not all published references were identified in this systematic review, albeit no additional references were retrieved from the bibliography cited in the articles located. Although we originally intended to perform a meta-analysis, this was not feasible because of methodological bias and heterogeneity between studies as regards the outcome and exposure definition, the time window considered, and the population in which the study was conducted. Nevertheless, during the elaboration of this manuscript, a meta-analysis including five studies analysed in this review was published [43]. The authors of the meta-analysis obtained an OR = 0.92 (95 % CI 0.8–1.0) and concluded that there is suggestive evidence that HB vaccination is not related with an increased risk of developing MS. However, regarding the study by Hernán et al. [19], the authors of the meta-analysis [43] selected the OR = 1.0 (95 % CI 0.5–2.1) instead of the OR = 3.1 (95 % CI 1.5–6.3); the former is less accurate, as it corresponds to the analysis using the date of diagnosis of MS, not the date of first symptoms, as the index date.
Conclusions
This review provides up-to-date information about the controversy concerning the relationship between HB vaccination and the risk of onset or exacerbation of MS. The findings of the review are mainly related to the difficulties in interpreting results from studies carried out with different methodologies and to limitations of the studies. It is difficult to conduct epidemiological studies in a complex scenario that must take into account not only the fact that individuals are exposed to multiple environmental factors (virus, other vaccines, etc.) during the course of their lives, but also the existence of certain major histocompatibility complex polymorphisms, which may determine the genetic predisposition to MS [4, 44]. Although cohort studies and nested case–control studies are adequate to address this association, several points must be considered to minimize bias. First, as evidence indicates long latency periods, a broad time window of study is needed. Consequently, the exposure assessment should never be based on interviews, and even vaccination certificates or written medical records are not recommended, as some may be missing over the years. Hence, information should be collected from large computerized health databases that exhaustively and prospectively record information about patients (such as vaccinations, drug therapies, certain lifestyle factors, medical diagnoses, etc.). As the vaccine has already been incorporated in immunization programmes in more than 150 countries, it is possible to obtain a representative sample of vaccinated subjects from the general population. Nevertheless, in order to include a representative sample of unvaccinated participants, the rate of vaccination in the general population should ideally not exceed 80 %. Moreover, there is a need for genetic and immunological studies (e.g. serological monitoring) [11], to help us to understand the mechanisms involved in CNS demyelinating diseases and to determine whether these conditions may be restricted to a predisposed subpopulation. These findings would enable decisions to be made regarding vaccination recommendations (or even vaccine development), which must be previously weighed against the benefits of HB prevention.
References
Stratton K, Almario DA, McCormick MC (2002) Immunization Safety review: hepatitis B vaccine and demyelinating neurological disorders. National Academies Press, Washington
Milo R, Kahana E (2010) Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 9:A387–A394
Maya R, Gershwin ME, Shoenfeld Y (2008) Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol 34:85–102
Piaggio E, Ben Younes A, Desbois S, Gout O, Tourbah A, Lyon-Caen O, Liblau RS (2005) Hepatitis B vaccination and central nervous system demyelination: an immunological approach. J Autoimmun 24:33–37
Ness JM, Bale JF (2009) Hepatitis vaccines and pediatric multiple sclerosis: does timing or type matter? Neurology 72:870–871
Zanetti AR, Van Damme P, Shouval D (2008) The global impact of vaccination against hepatitis B: a historical overview. Vaccine 26:6266–6273
Vial T, Descotes J (2004) Autoimmune diseases and vaccinations. Eur J Dermatol 14:86–90
Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D (2005) A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol 12:217–224
Faure E (2005) Multiple sclerosis and hepatitis B vaccination: could minute contamination of the vaccine by partial hepatitis B virus polymerase play a role through molecular mimicry? Med Hypotheses 65:509–520
Waisbren BA Sr (2008) Acquired autoimmunity after viral vaccination is caused by molecular mimicry and antigen complimentarity in the presence of an immunologic adjuvant and specific HLA patterns. Med Hypotheses 70:346–348
Salemi S, D’Amelio R (2010) Could autoimmunity be induced by vaccination? Int Rev Immunol 29:247–269
Zipp F, Weil JG, Einhäupl KM (1999) No increase in demyelinating diseases after hepatitis B vaccination. Nat Med 5:964–965
Sadovnick AD, Scheifele DW (2000) School-based hepatitis B vaccination programme and adolescent multiple sclerosis. Lancet 355:549–550
Touzé E, Gout O, Verdier-Taillefer MH, Lyon-Caen O, Alpérovitch A (2000) The first episode of central nervous system demyelinization and hepatitis B virus vaccination. Rev Neurol (Paris) 156:242–246
Ascherio A, Zhang SM, Hernán MA, Olek MJ, Coplan PM, Brodovicz K, Walker AM (2001) Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med 344:327–332
Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S, for the Vaccines in Multiple Sclerosis Study Group (2001) Vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med 344:319–326
Touzé E, Fourrier A, Rue-Fenouche C, Rondé-Oustau V, Jeantaud I, Bégaud B, Alpérovitch A (2002) Hepatitis B vaccination and first central nervous system demyelinating event: a case-control study. Neuroepidemiology 21:180–186
DeStefano F, Verstraeten T, Jackson LA, Okoro CA, Benson P, Black SB, Shinefield HR, Mullooly JP, Likosky W, Chen RT, for the Vaccine Safety Datalink Research Group (2003) Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol 60:504–509
Hernán MA, Jick SS, Olek MJ, Jick H (2004) Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. Neurology 63:838–842
Özakbas S, Idiman E, Yulug B, Pakoz B, Bahar H, Gulay Z (2006) Development of multiple sclerosis after vaccination against hepatitis B: a study based on human leucocyte antigen haplotypes. Tissue Antigens 68:235–238
Mikaeloff Y, Caridade G, Rossier M, Suissa S, Tardieu M (2007) Hepatitis B vaccination and the risk of childhood-onset multiple sclerosis. Arch Pediatr Adolesc Med 161:1176–1182
Mikaeloff Y, Caridade G, Assi S, Tardieu M, Suissa S, on behalf of the KIDSEP study group of the French Neuropaediatric Society (2007) Hepatitis B vaccine and risk of relapse after a first childhood episode of CNS inflammatory demyelination. Brain 130:1105–1110
Ramagopalan SV, Valdar W, Dyment DA, DeLuca GC, Yee IM, Giovannoni G, Ebers GC, Sadovnick AD, for the Canadian Collaborative Study Group (2009) Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiology 32:257–262
Mikaeloff Y, Caridade G, Suissa S, Tardieu M (2009) Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood. Neurology 72:873–880
Buttinelli C, Salvetti M, Ristori G (2001) Vaccinations and multiple sclerosis. N Engl J Med 344:1794
Naismith RT, Cross AH (2004) Does the hepatitis B vaccine cause multiple sclerosis? Neurology 63:772–773
WHO Global Advisory Committee on Vaccine Safety (2004) Response to the paper by MA Hernán and others in Neurology 14th September 2004 issue entitled “Recombinant Hepatitis B Vaccine and the Risk of Multiple Sclerosis”. http://www.who.int/vaccine_safety/topics/hepatitisb/multiple_sclerosis/sep_04/en/. Accessed 25 May 2011
Bégaud B, Alpérovitch A (2001) Vaccinations and multiple sclerosis. N Engl J Med 344:1793
Sturkenboom MC, Fourrier A (2001) Vaccinations and multiple sclerosis. N Engl J Med 344:1794
Hernán MA, Jick SS (2006) Hepatitis B vaccination and multiple sclerosis: the jury is still out. Pharmacoepidemiol Drug Saf 15:653–655
Gout O (2001) Vaccinations and multiple sclerosis. N Engl J Med 344:1794
Zuckerman JN (2006) Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol 78:169–177
Lièvre M, Members of Epidemiology Working Group of French Pharmacovigilance Commission, Costagliola D, Evans S, Fourrier A, Imbs JL, Levy-Bruhl D, Merle L, Micallef J, Oger E (2009) Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood. Neurology 73:1426–1427
Rutschmann OT, McCrory DC, Matchar DB, and the Immunization Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines (2002) Immunization and MS: a summary of published evidence and recommendations. Neurology 59:1837–1843
Demicheli V, Rivetti A, Di Pietrantonj C, Clements CJ, Jefferson T (2003) Hepatitis B vaccination and multiple sclerosis: evidence from a systematic review. J Viral Hepat 10:343–344
Schattner A (2005) Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine 23:3876–3886
Soubeyrand B, Boisnard F, Bruel M, Debois H, Delattre D, Gauthier A, Soum S, Thébault C (2000) Central nervous system demyelinating disease following hepatitis B vaccination with GenHevac B. Review of ten years of spontaneous notifications (1989–1998)]. Presse Med 29:775–780
Geier MR, Geier DA (2004) A case-series of adverse events, positive re-challenge of symptoms, and events in identical twins following hepatitis B vaccination: analysis of the Vaccine Adverse Event Reporting System (VAERS) database and literature review. Clin Exp Rheumatol 22:749–755
Geier DA, Geier MR (2005) A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity 38:295–301
Jefferson T, Traversa G (2002) Hepatitis B vaccination: risk-benefit profile and the role of systematic reviews in the assessment of causality of adverse events following immunisation. J Med Virol 67:451–453
Braillon A, Dubois G (2009) Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood. Neurology 72:2053
WHO (2008) WHO Statistical Information System. http://apps.who.int/whosis/data/. Accessed 8 June 2011
Farez MF, Correale J (2011) Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol 258:1197–1206
Selmi C, Battezzati PM, Gershwin ME, Tishler M, Shoenfeld Y (2005) Vaccines in the 21st century: the genetic response and the innocent bystander. Autoimmun Rev 4:79–81
Acknowledgments
We thank Prof. Florencio M. Ubeira (Departamento de Microbiología y Parasitología, Facultad de Farmacia, Santiago de Compostela, Spain) for critically reading the manuscript and his valuable comments.
Conflicts of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Sernández, V., Figueiras, A. Central nervous system demyelinating diseases and recombinant hepatitis B vaccination: a critical systematic review of scientific production. J Neurol 260, 1951–1959 (2013). https://doi.org/10.1007/s00415-012-6716-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6716-y