Abstract
Purpose
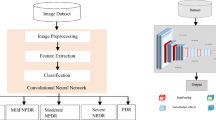
With the aging population and the global diabetes epidemic, the prevalence of age-related macular degeneration (AMD) and diabetic macular edema (DME) diseases which are the leading causes of blindness is further increasing. Intravitreal injections with anti-vascular endothelial growth factor (anti-VEGF) medications are the standard of care for their indications. Optical coherence tomography (OCT), as a noninvasive imaging modality, plays a major part in guiding the administration of anti-VEGF therapy by providing detailed cross-sectional scans of the retina pathology. Fully automating OCT image detection can significantly decrease the tedious clinician labor and obtain a faithful pre-diagnosis from the analysis of the structural elements of the retina. Thereby, we explore the use of deep transfer learning method based on the visual geometry group 16 (VGG-16) network for classifying AMD and DME in OCT images accurately and automatically.
Method
A total of 207,130 retinal OCT images between 2013 and 2017 were selected from retrospective cohorts of 5319 adult patients from the Shiley Eye Institute of the University of California San Diego, the California Retinal Research Foundation, Medical Center Ophthalmology Associates, the Shanghai First People’s Hospital, and the Beijing Tongren Eye Center, with 109,312 images (37,456 with choroidal neovascularization, 11,599 with diabetic macular edema, 8867 with drusen, and 51,390 normal) for the experiment. After images preprocessing, 1000 images (250 images from each category) from 633 patients were selected as validation dataset while the rest images from another 4686 patients were used as training dataset. We used deep transfer learning method to fine-tune the VGG-16 network pre-trained on the ImageNet dataset, and evaluated its performance on the validation dataset. Then, prediction accuracy, sensitivity, specificity, and receiver-operating characteristic (ROC) were calculated.
Results
Experimental results proved that the proposed approach had manifested superior performance in retinal OCT images detection, which achieved a prediction accuracy of 98.6%, with a sensitivity of 97.8%, a specificity of 99.4%, and introduced an area under the ROC curve of 100%.
Conclusion
Deep transfer learning method based on the VGG-16 network shows significant effectiveness on classification of retinal OCT images with a relatively small dataset, which can provide assistant support for medical decision-making. Moreover, the performance of the proposed approach is comparable to that of human experts with significant clinical experience. Thereby, it will find promising applications in an automatic diagnosis and classification of common retinal diseases.









Similar content being viewed by others
References
Mariotti SP (2012) Global data on visual impairments 2010. http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf . Accessed 6 Jun 2018
Bourne RRA, Flaxman SR, Braithwaite T et al (2017) Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health 5:e888–e897. https://doi.org/10.1016/S2214-109X(17)30293-0 Accessed 6 Jun 2018
Bourne RRA, Jonas JB, Flaxman SR et al (2014) Vision loss expert Group of the Global Burden of Disease Study, prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol 98:629–638. https://doi.org/10.1136/bjophthalmol-2013-304033
Pedro RA (2013) Current status in diabetic macular edema treatments. World J Diabetes 4:165–169. https://doi.org/10.4239/wjd.v4.i5.165
Koh JEW, Ng EYK, Bhandary SV et al (2018) Automated retinal health diagnosis using pyramid histogram of visual words and fisher vector techniques. Comput Biol Med 92:204–209. https://doi.org/10.1016/j.compbiomed.2017.11.019
Ferrara N (2010) Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. https://doi.org/10.1038/nm1010-1107 . Accessed 6 Jun 2018
Varma R, Bressler NM, Doan QV et al (2014) Prevalence of and risk factors for diabetic macular edema in the United States. Jama Ophthalmol 132:1334–1340. https://doi.org/10.1001/jamaophthalmol.2014.2854
Brown DM, Kaiser PK, Michels M et al (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. https://doi.org/10.1056/NEJMoa062655
Keane PA, Patel PJ, Liakopoulos S et al (2012) Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 57:389–414. https://doi.org/10.1016/j.survophthal.2012.01.006
Abràmoff MD, Garvin MK, Sonka M (2010) Retinal imaging and image analysis. IEEE Rev Biomed Eng 3:169–208. https://doi.org/10.1109/RBME.2010.2084567
Drexler W, Fujimoto JG (2008) State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res 27:45–88. https://doi.org/10.1016/j.preteyeres.2007.07.005
Bressler NM (2002) Early detection and treatment of neovascular age-related macular degeneration. http://www.jabfm.org/content/15/2/142.full.pdf . Accessed 6 Jun 2018
Ciulla TA, Amador AG, Zinman B (2003) Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. http://care.diabetesjournals.org/content/diacare/26/9/2653.full.pdf . Accessed 6 Jun 2018
Srinivasan PP, Kim LA, Mettu PS et al (2014) Fully automated detection of diabetic macular edema and dry age-related macular degeneration from optical coherence tomography images. Biomedical optics express 5:3568–3577. https://doi.org/10.1364/BOE.5.003568
Liu YY, Chen M, Ishikawa H et al (2011) Automated macular pathology diagnosis in retinal OCT images using multi-scale spatial pyramid and local binary patterns in texture and shape encoding. Med Image Anal 15:748–759. https://doi.org/10.1016/j.media.2011.06.005
Alsaih K, Lemaître G, Vall JM et al (2016) Classification of sd-oct volumes with multi pyramids, lbp and hog descriptors: application to dme detections. https://doi.org/10.1109/EMBC.2016.7590956 . Accessed 6 Jun 2018
Abràmoff MD, Lou Y, Erginay A et al (2016) Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 57:5200–5206. https://doi.org/10.1167/iovs.16-19964
Schlegl T, Waldstein SM, Bogunovic H et al (2017) Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology 125:549–558. https://doi.org/10.1016/j.ophtha.2017.10.031
Burlina P, Freund DE, Joshi N et al (2016) Detection of age-related macular degeneration via deep learning. https://doi.org/10.1109/ISBI.2016.7493240 . Accessed 6 Jun 2018
Perona P, Malik J (1990) Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell 12:629–639. https://doi.org/10.1109/34.56205
Lee JG, Jun S, Cho YW et al (2017) Deep learning in medical imaging: general overview. Korean J Radiol 18:570–584. https://doi.org/10.3348/kjr.2017.18.4.570
Zhao B, Lu H, Chen S et al (2017) Convolutional neural networks for time series classification. J Syst Eng Electron 28:162–169. https://doi.org/10.21629/JSEE.2017.01.18
Kermany DS, Goldbaum M, Cai W et al (2018) Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 172:1122–1131.e9. https://doi.org/10.1016/j.cell.2018.02.010
Funding
This work was supported by the National Key Research and Development Program of China (2016YFF0101400) and the National Natural Science Foundation of China (51675321).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. For this type of study, formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, F., Chen, H., Liu, Z. et al. Fully automated detection of retinal disorders by image-based deep learning. Graefes Arch Clin Exp Ophthalmol 257, 495–505 (2019). https://doi.org/10.1007/s00417-018-04224-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-04224-8