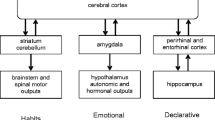
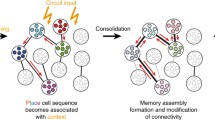
Abstract
Since the cell assembly (CA) was hypothesised, it has gained substantial support and is believed to be the neural basis of psychological concepts. A CA is a relatively small set of connected neurons, that through neural firing can sustain activation without stimulus from outside the CA, and is formed by learning. Extensive evidence from multiple single unit recording and other techniques provides support for the existence of CAs that have these properties, and that their neurons also spike with some degree of synchrony. Since the evidence is so broad and deep, the review concludes that CAs are all but certain. A model of CAs is introduced that is informal, but is broad enough to include, e.g. synfire chains, without including, e.g. holographic reduced representation. CAs are found in most cortical areas and in some sub-cortical areas, they are involved in psychological tasks including categorisation, short-term memory and long-term memory, and are central to other tasks including working memory. There is currently insufficient evidence to conclude that CAs are the neural basis of all concepts. A range of models have been used to simulate CA behaviour including associative memory and more process- oriented tasks such as natural language parsing. Questions involving CAs, e.g. memory persistence, CAs’ complex interactions with brain waves and learning, remain unanswered. CA research involves a wide range of disciplines including biology and psychology, and this paper reviews literature directly related to the CA, providing a basis of discussion for this interdisciplinary community on this important topic. Hopefully, this discussion will lead to more formal and accurate models of CAs that are better linked to neuropsychological data.





Similar content being viewed by others
Notes
An atomic CA is not composed of other CAs.
Statistical mechanics can be used to describe CAs, with a set of firing neurons being an attractor basin. The Hopfield model (Hopfield 1982 and see Sect. 5.1.3) is a particularly good example of attractor basins with a particular set of neurons firing and then continuing to fire in response to a particular input. The firing pattern moves from the initial input state, down an energy slope to a new firing state, which has attracted the activity.
If a language is Turing complete, anything that can be programmed can be programmed in it. So, to some degree, CAs are equivalent to Java.
References
Abbott L (1999) Lapicque’s introduction of the integrate-and-fire model neuron (1907). Brain Res 50:303–304
Abbott L, Nelson S (2000) Synaptic plasticity: taming the beast. Nat Neurosci 3:1178–1183
Abeles M, Bergman H, Margalit E, Vaadia E (1993) Spatiotemporal firing patterns in the frontal cortex of behaving monkeys. J Neurophysiol 70(4):1629–1638
Ackley D, Hinton G, Sejnowski T (1985) A learning algorithm for boltzmann machines. Cogn Sci 9:147–169
Amari S (1977) Neural theory of association and concept-formation. Biol Cybern 26:175–185
Amit D (1989) Modelling brain function: the world of attractor neural networks. Cambridge University Press, Cambridge
Amit D, Brunel N (1995) Learning internal representations in an attractor neural network with analogue neurons. Netw Comput Neural Syst 6:359–388
Anderson J, Lebiere C (1998) The atomic components of thought. Lawrence Erlbaum, New Jersey
Assad W, Rainer G, Miller E (2000) Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol 84:451–459
Auseng P, Grismayr B, Freunberger R, Klimesch W (2010) Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev 34:1015–1022
Averbeck B, Latham P, Pouget A (2006a) Neural correlations, population coding and computation. Nat Rev Neurosci 7:358–366
Averbeck B, Sohn J, Lee D (2006b) Activity in prefrontal cortex during dynamic selection of action sequences. Nat Neurosci 9(2): 276–282
Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4:829–839
Baeg E, Kim Y, Kim J, Ghim J, Kim J, Jung M (2007) Learning-induced enduring changes in functional connectivity among prefrontal cortical neurons. J Neurosci 27(4):909–918
Barlow H (1972) Single units and sensation: a neuron doctrine for perceptual psychology? Perception 1:371–394
Belavkin R, Huyck C (2010) Conflict resolution and learning probability matching in a neural cell-assembly architecture. Cogn Syst Res 12:93–101
Bendor D, Wang X (2008) Neural response properties of primary, rostral, and rostraltemporal core fields in the auditory cortex of marmoset monkeys. J Neurophysiol 100:888–906
Bennett B, Callaway J, Wilson C (2000) Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci 20(22):8493–8503
Bertschinger N, Natschlager T (2004) Real-time computation at the edge of chaos in recurrent neural networks. Neural Comput 16(7):1413–1436
Beurle R (1956) Properties of a mass of cells capable of regenerating pulses. Trans R Soc Lond B 240:55–94
Bevan M, Wilson C (1999) Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci 19:7617–7628
Bi G, Poo M (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18(24):10464–10472
Bichot N, Schall J (1999) Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci 2(6):549–554
Bieser A, Muller-Preuss P (1996) Auditory responsive cortex in the squirrel monkey: neural responses to amplitude-modulated sounds. Exp Brain Res 108:273–284
Bisley J, Zaksas D, Droll J, Pasternak T (2004) Activity of neurons in cortical area MT during a memory for motion task. J Neurophysiol 91:286–300
Bogacz R (2007) Optimal decision network with distributed representation. Neural Netw 20:564–576
Booth J, Burman D, Meyer J, Gitelman D, Parrish T (2004) Development of brain mechanisms for processing orthographic and phonologic representations. J Cogn Neurosci 16(7):1234–1249
Braitenberg V (1978) Cell assemblies in the cerebral cortex. In: Heim R, Palm G (eds) Theoretical Approaches to Complex Systems. Lecture Notes in Biomathematics, vol 21. Springer, Berlin, pp 171–188
Braitenberg V (1989) Some arguments for a theory of cell assemblies in the cerebral cortex. In: Nadel C, Culicover H (eds) Neural connections, mental computation. MIT Press, Cambridge
Brecht M, Schneider M, Sakmann B, Margrie T (2004) Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427:704–710
Bressler S (1995) Large-scale cortical networks and cognition. Brain Res Rev 20:288–304
Brette R, Rudolph M, Carnevale T, Hines M, Beeman D, Bower J, Diesmann M, Morrison A, Goodman P, Harris F, Zirpe M, Natschalager T, Pecevski D, Ermentrout B, Djurfeldt M, Lansner A, Rochel O, Vieville T, Muller E, Dafison A, ElBoustani S, Destexhe A (2007) Simulation of networks of spiking neurons: a review of tools and strategies. J Comput Neurosci 23:349–398
Bruno R, Sakmann B (2006) Cortex is driven by weak by synchronously active thalamocortical synapses. Science 312:1622–1627
Buckner R, Wheeler M (2001) The cognitive neuroscience of remembering. Nat Rev Neurosci 2:624–634
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Buonomano D, Merzenich M (1998) Cortical plasticity: from synapses to maps. Ann Rev Neurosci 21:149–186
Burgess N, Hitch G (2005) Computational models of working memory: putting long-term memory into context. Trends Cogn Sci 9(11):535–541
Burkitt A (2006) A review of the integrate-and-fire neuron model: I. homogeneous synaptic input. Biol Cybern 95(1):1–19
Butt S, Harris-Warrick R, Kiehn O (2002) Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci 22(22):9961–9971
Buzaski G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929
Byrne E, Huyck C (2010) Processing with cell assemblies. Neurocomputing 74:76–83
Cho J, Sharp P (2001) Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci 115(1):3–25
Christian K, Thompson R (2003) Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem 10:427–455
Chrobak J, Buzaski G (1998) Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci 18(1):388–398
Churchland P, Sejnowski T (1999) The computational brain. MIT Press, Cambridge
Constantinidis C, Procyk E (2004) The primate working memory networks. Cogn Affect Behav Neurosci 4(4):444–465
Cossart R, Aronov D, Yuste R (2003) Attractor dynamics of network up states in the neocortex. Nature 423:283–288
Cowan N (1988) Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol Bull 1–4(2):163–191
Crowe D, Averbeck B, Chafee M (2008) Neural ensemble decoding reveals a correlate of viewer-to-object-centered spatial transformation in monkey parietal cortex. J Neurosci 28(20):5218–5228
Crutcher M, Russo G, Ye S, Backus D (2004) Target-, limb-, and context-dependent neural activity in cingulate and supplementary motor areas of the monkey. Exp Brain Res 158:278–288
Curtis C, D’Esposito M (2003) Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7(9):415–423
Deco G, Rolls E (2005) Sequential memory: a putative neural and synaptic dynamical mechanism. J Cogn Neurosci 17(2):294–307
Desimone R, Albright T, Gross C, Bruce C (1984) Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci 4(8):2051–2062
D’Esposito M (2007) From cognitive to neural models of working memory. Philos Trans R Soc 362:761–772
deVries P (2004) Effects of binning in the identification of objects. Psychol Res 69:41–66
Diester I, Nider A (2007) Semantic associations between signs and numerical categories in prefrontal cortex. PLOS Biol 5(11):2684–2695
Douglas R, Martin K (1991) A functional microcircuit for cat visual cortex. J Physiol 440:735–769
Douglas R, Martin K (2004) Neuronal circuits of the neocortex. Ann Rev Neurosci 27:419–451
Dragoi G, Buzaski G (2006) Temporal encoding of place sequences in hippocampal cell assemblies. Neuron 50:145–157
Dragoi G, Tonegawa S (2011) Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469:397–401
Durstewitz D, Seamans J, Sejnowski T (2000) Neurocomputational models of working memory. Nat Neurosci Suppl 3:1184–1191
Egorov A, Hamam B, Fransen E, Hasselmo M, Alonso A (2002) Graded persistent activity in entorhinal cortex neurons. Nature 420:173–178
Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50
Eliasmith C, Thagard P (2001) Integrating structure and meaning: a distributed model of analogical mapping. Cogn Sci 25:245–286
Engel A, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2:704–716
Engel R, Tuholski S, Laughlin J, Conway A (1999) Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol 12(3):309–331
Field D (1994) What is the goal of sensory coding. Neural Comput 6:559–601
Fischer B, Boch R, Bach M (1981) Stimulus versus eye movements: comparison of neural activity in the striate and prelunate visual cortex (a17 and a19) of trained rhesus monkey. Exp Brain Res 43:69–77
Fransen E, Lansner A, Liljenstrom H (1992) A model of cortical associative memory based on hebbian cell assemblies. In: Niklasson L, Boden M (eds) Connectionism in a broad perspective. Ellis Horwood, London; Springer, Berlin, pp 165–171
Freedman D, Assad J (2006) Experience-dependent representation of visual categories in parietal cortex. Nature 433:85–88
Freiwald W, Kreiter A, Singer W (2001) Synchronization and assembly formation in the visual cortex. In: Nicolelis M (ed) Progress in brain research, vol 130. Elsevier, Amsterdam
Fujii H, Hiroyuke I, Aihara K, Ichinose N, Tsukada M (1996) Dynamical cell assembly hypothesis—theoretical possibility of spatio-temporal coding in the cortex. Neural Netw 9(8):1303–1350
Funahashi S (2001) Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res 39:147–165
Fusi S (2008) A quiescent working memory. Science 319:1495–1496
Fuster J, Alexander G (1971) Neuron activity related to short-term memory. Science 173:652–654
Fuster J, Bodner M, Kroger J (2000) Cross-modal and cross-temporal association in neurons of frontal cortex. Nature 405:347–351
Fyfe C (2005) Hebbian learning and negative feedback networks. Springer, Berlin
Gallese V, Lakoff G (2005) The brain’s concepts: the role of the sensory motor system in conceptual knowledge. Cogn Neuropsychol 22(3/4):455–479
Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996) Action recognition in the premotor cortex. Brain 119:593–609
Gargnani M, Wennekers T, Pulvermuller F (2007) A neuronal model of the language cortex. Neurocomputing 70:1914–1919
Gazzaley A, Rissman J, D’Esposito M (2004) Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci 4(4):580–599
Georgopoulos A, Schwartz A, Kettner R (1986) Neuronal populations coding of movement direction. Science 233:1416–1419
Gerstner W, Kistler W (2002) Mathematical formulations of hebbian learning. Biol Cybern 87:404–415
Gochin P, Colombo M, Dorfman G, Gerstein G, Gross C (1994) Neural ensemble coding in inferior temporal cortex. J Neurophysiol 71(6):2325–2337
Golari G, Ghahremani D, Whitfield-Gabrieli S, Reiss A, Eberhardt J, Gabrieli J, Grill-Spector K (2007) Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci 10(4):512–522
Goldman-Rakic P (1995) Cellular basis of working memory. Neuron 14:477–485
Goldman-Rakic P (1996) Regional and cellular fractionation of working memory. PNAS 93:13473–13480
Gray C, Singer W (1989) Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. PNAS 86:1698–1702
Grill-Spector K, Golarai G, Gabrieli J (2008) Developmental neuroimaging of the human ventral visual cortex. Trends Cogn Sci 12:152–162
Grinvald A, Arieli A, Tsodyks M, Kenet T (2003) Neuronal assemblies: single cortical neurons are obedient members of a huge orchestra. Biopolymers 68:422–436
Gutierrez R, Carmena J, Nicolelis M, Simon S (2006) Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol 95:119–133
Harnad S (1990) The symbol grounding problem. Phys D 42:335–346
Harris K (2005) Neural signatures of cell assembly organization. Nat Rev Neurosci 6:399–407
Harris K, Csicsvari J, Hirase H, Dragoi G, Buzsaki G (2003) Organization of cell assemblies in the hippocampus. Nature 424: 552–556
Harrison S, Tong F (2009) Decoding reveals the contents of visual working memory in early visual areas. Nature 458:632–635
Hebb DO (1949) The organization of behavior: a neuropsychological theory. Wiley, New York
Hempel C, Hartman K, Wang X, Turrigiano G, Nelson S (2000) Multiple forms of short-term plasticity at excitatory in rat medial prefrontal cortex. J Neurophysiol 83:3031–3041
Henson R, Rugg M, Shallice T, Josephs O, Dolan R (1999) Recollection and familiarity in recognition in memory: an event-related functional magnetic resonance imaging study. J Neurosci 19(10): 3962–3972
Hetherington P, Shapiro M (1993) Simulating hebb cell assemblies: the necessity for partitioned dendritic trees and a post-net-pre ltd rule. Netw Comput Neural Syst 4:135–153
Histed M, Pasupathy A, Miller E (2009) Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron 63(2):244–253
Hochberg L, Serruya M, Friehs G, Mukand J, Saleh M, Caplan A, Branner A, Chen D, Penn R, Dooghue J (2006) Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442(13):164–171
Hodgkin A, Huxley A (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117:500–544
Hofstadter D (1979) Godel, escher and bach: an eternal golden braid. Basic Books, New York
Hopfield J (1982) Neural nets and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA 79:2554–2558
Hoshino O, Miyamoto M, Zheng M, Kuroiwa K (2002) A neural network model for encoding and perception of vowel sounds. Neurocomputing 44–46:435–442
Howard M, Volkov I, Mirsky R, Garell P, Noh M, Granner M, Damasio H, Steinschneider M, Reale R, Hind J, Brugge J (2000) Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol 416:79–92
Hubel D, Wiesel T (1962) Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol 160:106–154
Humphries M, Wood R, Gurney K (2009) Dopamine-modulated dynamic cell assemblies generated by the GABAergic striatal microcircuit. Neural Netw 22:1174–1188
Huyck C (2007) Creating hierarchical categories using cell assemblies. Connect Sci 19(1):1–24
Huyck C (2009) A psycholinguistic model of natural language parsing implemented in simulated neurons. Cogn Neurodyn 3(4):316–330
Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R (2004) Synfire chains and cortical songs: temporal modules of cortical activity. Science 304(23):559–564
Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T (2009) Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci 12(12):1586–1593
Ito M (1989) Long-term depression. Ann Rev Neurosci 12:85–102
Iyer L, Doboli S, Minai A, Brown V, Levine D, Paulus P (2009) Neural dynamics of idea generation and the effects of priming. Neural Netw 22:674–686
Izhikevich E (2004) Which model to use for cortical spiking neurons? IEEE Trans Neural Netw 15(5):1063–1070
Jackendoff R (2002) Foundations of language: brain, meaning, grammar, evolution. Oxford University Press, Oxford
James W (1892) Psychology: the briefer course. University of Notre Dame Press, USA
Jezek K, Henriksen E, Treves A, Moser E, Moser M (2011) Theta-paced flickering between place-cell maps in the hippocampus. Nature 478:246–249
Jonides J, Smith E, Koeppe R, Awh E, Minoshima S, Mintun M (1993) Spatial working memory in humans as revealed by pet. Nature 363:623–625
Just M, Chherkassky V, Aryal S, Mitchell T (2010) A neurosemantic theory of concrete noun representation based on the underlying brain code. PLoS ONE 5(1):e8622
Kaplan S, Weaver M, French R (1990) Active symbols and internal models: towards a cognitive connectionism. AI Soc 4:51–71
Kaplan S, Sontag M, Chown E (1991) Tracing recurrent activity in cognitive elements (trace): a model of temporal dynamics in a cell assembly. Connect Sci 3:179–206
Katz D, Simon S, Nicolelis M (2002) Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J Neurosci 22(5):1850–1857
Keri S (2003) The cognitive neuroscience of category learning. Brain Res Rev 43:85–109
Klausberger T, Magill P, Marton L, Roberts J, Cobden P, Buzaski G, Somogyi P (2003) Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421:844–848
Kleinsmith L, Kaplan S (1963) Paired-associate learning as a function of arousal and interpolated interval. J Exp Psychol 65(2): 190–193
Knoblauch A, Markert H, Palm G (2004) An associative model of cortical language and action processing. In: Proceedings of the ninth neural computation and psychology workshop
Knoblauch A, Kupper R, Gewaltig M, Korner U, Korner E (2007) A cell assembly based model for the cortical microcircuitry. Neurocomputing 70:1838–1842
Krahe R, Gabbiani F (2004) Burst firing in sensory systems. Nat Rev Neurosci 5:13–24
Kreiman G, Koch C, Fried I (2000) Imagery neurons in the human brain. Nature 408:357–361
Kreiter A, Singer W (1996) Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. J Neurosci 16(7):2381–2396
Kuo C, Chiou R, Liang K, Yen C (2009) Differential involvement of the anterior cingulate and primary sensorimotor cortices in sensory and affective functions of pain. J Neurophysiol 101:1201–1210
Lamme V, Super H, Spekreijse H (1998) Feedforward, horizontal, and feedback processing in the visual cortex. Curr Opin Biol 8:525–535
Lansner A (2009) Associative memory models: from the cell-assembly theory to biophysically detailed cortex simulations. Trends Neurosci 32(3):178–186
Lapish C, Durstewitz D, Chandler L, Seamans J (2008) Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. PNAS 105:33:11963–11968
Larimer P, Strowbridge B (2009) Representing information in cell assemblies: persistent activity mediated by semilunar granule cells. Nat Neurosci 13:213–222
Laubach M, Wessberg J, Nicolelis M (2000) Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature 405:567–570
LeBihan D, Turner R, Zeffiro T, Cuenod C, Jezzard P, Bonnerot V (1993) Activation of human primary visual cortex during visual recall: a magnetic resonance imaging study. PNAS 90:11802–11805
Levy N, Horn D, Meilijson I, Ruppin E (2001) Distributed synchrony in a cell assembly of spiking neurons. Neural Netw 14:815–824
Liebenthal E, Uhlmann O, Camhi J (1994) Critical parameters of the pike trains in a cell assembly: coding of turn direction by giant interneurons of the cockroach. J Comp Physiol A 174:281–296
Logothetis N, Pauls J, Poggio T (1995) Shape representation in the inferior temporal cortex of monkeys. Curr Biol 5(5):552–563
Lundqvist M, Rehn M, Djurfeldt M, Lansner A (2006) Attractor dynamics in a modular neural network model of neocortex. Netw Comput Neural Syst 17:253–276
Macaluso E, Frith C, Driver J (2000) Modulation of human visual cortex by crossmodal spatial attention. Science 289:1206–1208
Malenka R, Nicoll R (1999) Long-term potentiation—a decade of progress? Science 285:1870–1874
Maquet P (2001) The role of sleep in learning and memory. Science 10:1048–1052
Markram H (2006) The blue brain project. Nat Rev Neurosci 7:153–160
Martini F (2001) Fundamentals of anatomy and physiology. Prentice Hall, New Jersey
Maunsell J, Van Essen D (1983) Functional properties of neurons in the middle temporal visual area of the macaque monkey. I. selectivity for stimulus direction, speed and orientation. J Neurophysiol 49(5):1127–1147
McCoy A, Platt M (2005) Risk-sensitive neurons in the macaque posterior cingulate cortex. Nat Neurosci 8(9):1220–1227
McCulloch W, Pitts W (1943) A logical calculus of ideas immanent in nervous activity. Bull Math Biophys 5:115–133
McIntosh A, Cabeza R, Lobaugh N (1998) Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. J Am Physiol Soc 90:2790–2796
McNaughton B, Battaglia F, Jensen O, Moser E, Moser M (2006) Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci 7:663–678
Miller E, Erickson C, Desimone R (1996) Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16(16):5154–5167
Milner P (1957) The cell assembly: mark II. Psychol Rev 64(4):242–252
Minsky M (1986) The society of mind. Simon and Schuster, New York
Miyake A, Friedman N, Emerson M, Witzki A, Howeter A, Wager T (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41:49–100
Mongillo G, Barak O, Tsodyks M (2008) Synaptic theory of working memory. Science 319:1543–1546
Moore C, Nelson S (1998) Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol 80:2882–2892
Morrison J, Hof P (1997) Life and death of neurons in the aging brain. Science 278:412–419
Nakamura K, Matsumoto K, Mikami A, Kubota K (1994) Visual response properties of single neurons in the temporal pole of behaving monkeys. J Neurophysiol 71(3):1206–1221
Nakamura K, Chung H, Graziano M, Gross C (1999) Dynamic representation of eye position in the parieto-occipital sulcus. J Neurophysiol 81:2374–2385
Naya Y, Sakai K, Miyashita Y (1996) Activity of primate inferotemporal neurons related to a sought target in pair-association task. PNAS 93:2664–2669
Nee D, Jonides J (2008) Neural correlates of access to short-term memory. PNAS 105:37:14228–14233
Nicolelis M, Lin R, Woodward D, Chapin J (1993) Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. PNAS 90:2212–2216
Nicolelis M, Baccala L, Lin R, Chapin J (1995) Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science 268:1353–1358
Nishimura M, Scherf S, Behrmann M (2009) Development of object recognition in humans. F1000 Biol Rep 1:56
Nitz D, Cowen S (2008) Crossing borders: sleep reactivation as a window on cell assembly formation. Nat Neurosci 11:126–128
O’Donnell P, Grace A (1995) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15:3622–3639
Ohl F, Scheich H, Freeman W (2001) Change in pattern of ongoing cortical activity with auditory category learning. Nature 412:733–736
Olufsen M, Whittington M, Camperi M, Kopell N (2003) New roles for the gamma rhythm: population tuning and preprocessing for the beta rhythm. Comput Neurosci 14:33–54
O’Neill J, Senior T, Allen K, Huxter J, Csicsvari J (2008) Reactivation of experience-dependent cell assembly patterns in hippocampus. Nat Neurosci 11:209–215
O’Reilly R (1999) Six principles for biologically-based computational models of cortical cognition. Trends Cogn Sci 2:455–462
O’Reilly R, Noelle D, Braver T, Cohen J (2002) Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex 12:62–101
Paller K (1997) Consolidating dispersed neocortical memories: the missing link in amnesia. Memory 5(1/2):73–88
Palm G, Sommer F (1995) Associative data storage and retrieval in neural networks. In: Domany E, van Hemmen J, Schulten K (eds) Models of neural networks III. Springer, Berlin
Pastalkova E, Itskov V, Amarsingham A, Buzaski G (2008) Internally generated cell assembly sequences in the rat hippocampus. Science 321:1322–1327
Pasternak T, Greenlee M (2005) Working memory in primate sensory systems. Nat Rev Neurosci 6:97–107
Pasupathy A, Connor C (2002) Population coding of shape in area v4. Nat Neurosci 5(12):1332–1338
Plate T (1995) Holographic reduced representations. IEEE Trans Neural Netw 6(3):623–641
Plenz D, Thiagarajan T (2007) The organizing principles of neuronal avalanches: cell assemblies in the cortex? Trends Neurosci 30(3):101–110
Pogio G, Fischer B (1977) Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkeys. J Neurosci 40(6):1392–1405
Ponzi A, Wickens J (1977) Input dependent cell assembly dynamics in a model of the striatal medium spiny neuron network. J Neurosci 40(6):1392–1405
Procyk E, Tanaka Y, Joseph J (2000) Anterior cingulate activity during routine and non-routine sequential behaviours in macaques. Nat Neurosci 3:502–508
Pulvermuller F (1999) Words in the brain’s language. Behav Brain Sci 22:253–336
Purushothaman G, Bradley D (2005) Neural population code for fine perceptual decision in area MT. Nat Neurosci 8(1):99–106
Qin Y, McNaughton B, Skaggs W, Barnes C (1997) Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos Trans R Soc B 352:1525–1533
Qiu F, von der Heydt R (2005) Figure and ground in the visual cortex: V2 combines stereoscopic cues with gestalt rules. Neuron 47:155–166
Quintana J, Fuster J (1999) From perception to action: temporal integrative functions of prefrontal and parietal neurons. Cereb Cortex 9:213–221
Quiroga R, Reddy L, Kreiman G, Koch C, Fried I (2005) Invariant visual representation by single neurons in the human brain. Nature 435:1102–1107
Raizada R, Grossberg S (2003) Towards a theory of the laminar architecture of cerebral cortex: computational clues from the visual system. Cereb Cortex 13(1):100–113
Ranganath C, Cohen M, Brozinsky C (2005) Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cogn Neurosci 17:994–1010
Reinagel P, Reid R (2000) Temporal coding of visual information in the thalamus. J Neurosci 20(14):5392–5400
Rennaker R, Chen C, Ruyle A, Sloan A, Wilson D (2007) Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci 27(7):1534–1542
Repa J, Muller J, Apergis J, Desrochers T, Zhou Y, LeDoux J (2001) Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci 4(7):724–731
Reynolds G, Richards J (2009) Cortical source localization of infant cognition. Dev Neuropsychol 34(3):312–329
Riches I, Wilson F, Brown M (1991) The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J Neurosci 11(6):1763–1779
Riesen A (1947) The development of visual perception in man and chimpanzee. Science 106:107–108
Rochester N, Holland J, Haibt L, Duda W (1956) Tests on a cell assembly theory of the action of the brain using a large digital computer. IRE Trans Inf Theory IT 2:80–93
Roelfsma P, Engel A, Konig P, Singer W (1997) Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature 385:157–161
Rolls E (2008) Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiolgica Hungarica 95(2):131–164
Rolls E, Inoue K, Browning A (2003) Activity of primate subgenual cingulate cortex neurons is related to sleep. J Neurophysiol 90:134–142
Romanski L, Averbeck B, Diltz M (2004) Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurphysiol 93:734–747
Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber M, Dold G, Hallett M (1996) Activation of primary visual cortex by braille reading in blind subjects. Nature 380:526–528
Sakai K, Miyashita Y (1991) Neural organization for the long-term memory of paired associates. Nature 354:152–155
Sakurai Y (1998a) Cell-assembly coding in several memory processes. Neurobiol Learn Mem 70:212–225
Sakurai Y (1998b) The search for cell assemblies in the working brain. Behav Brain Res 91:1–13
Sakurai Y, Tuakahashi S, Inoue M (2004) Stimulus duration in working memory is represented by neuronal activity in the monkey prefrontal cortex. Eur J Neurosci 20:1069–1080
Salihoglu U, Bersini H, Yamaguchi Y, Molter C (2009) Online unsupervised formation of cell assemblies for the encoding of multiple cognitive maps. Neural Netw 22:687–696
Scherberger H, Jarvis M, Anderson R (2005) Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron 46:347–354
Schoenbaum G (1998) Cell assemblies and the ghost in the machine. In: Eichenbaum H, Davis J (eds) Neuronal ensembles, strategies for recording and decoding, Wiley, New York, pp 81–116
Seamans J, Nogueira L, Lavin L (2003) Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cereb Cortex 13:1242–1250
Setola P, Reilly R (2005) Words in the brain’s language: an experimental investigation. Brain Lang 94:251–259
Shoham D, Glaser D, Arieli A, Kenet T, Wijnbergen C, Toledo Y, Hildesheim R, Grinvald A (1999) Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron 24:791–802
Siegel G, Carter C, Thase M (2006) Use of fmri to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry 163:735–738
Sigala N, Gabbiani F, Logothetis N (2002) Visual categorization and object representation in monkeys and humans. J Cogn Neurosci 14(2):187–198
Sigala N, Kusunoki M, Nimmo-Smith I, Gaffan D, Duncan J (2008) Hierarchical coding for sequential task events in the monkey prefrontal cortex. PNAS Neurosci 105:33:11969–11974
Silvanto J, Muggleton N (2008) New light through old windows: moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. NeuroImage 39:549–552
Singer W, Engel A, Kreiter A, Munk M, Neuenschwander S, Roelfsema P (1997) Neuronal assemblies: necessity, signature and detectability. Trends Cogn Sci 1(7):252–261
Smith K (2010) Settling the great glia debate. Nature 468:160–162
Sougne J (2001) Binding and multiple instantiation in a distributed network of spiking neurons. Connect Sci 13:99–126
Stackman R, Taube J (1998) Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci 18(21):9020–9037
Steriade M, Nunez A, Amzica F (1993) A novel slow (\(<\)1 hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13(8):3252–3265
Stern E, Jaeger D, Wilson C (1998) Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature 394:475–478
Sutherland G, McNaughton B (2000) Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol 10:180–186
Taddeo M, Floridi L (2005) Solving the symbol grounding problem: a critical review of fifteen years of research. J Exp Theor Artif Intell 17(4):419–445
Talk A, Kang E, Gabriel M (2004) Independent generation of theta rhythm in the hippocampus and posterior cingulate cortex. Brain Res 1015:15–24
Tark K, Curtis C (2009) Persistent neural actcivity in the human frontal cortex when maintaining space that is off the map. Nat Neurosci 12:1463–1468
Terman D, Wang D (1995) Global competition and local cooperation in a network of neural oscillators. Phys D 81:148–176
Tudusciuc O, Nieder A (2007) Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. PNAS 104(36):14513–14518
Usher M, Donnelly N (1998) Visual synchrony affects binding and segmentation in perception. Nature 394:179–182
Valiant L (2005) Memorization and association on a realistic neural model. Neural Comput 17:527–555
van der Velde F, de Kamps M (2006) Neural blackboard architectures of combinatorial structures in cognition. Behav Brain Sci 29:1–72
von der Malsburg C (1981) The correlation theory of brain function. Technical Report, Department of Neurobiology, Max-Planck-Institute for Biophyscial Chemistry
Wallace D, Kerr J (2010) Chasing the cell assembly. Curr Opin Neurobiol 20:296–305
Wallis J, Anderson K, Miller E (2001) Single neurons in prefrontal cortex encode abstract rules. Nature 411:953–956
Wang X (2001) Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 24(8):455–463
Wang Y, Markram H, Goodman P, Berger T, Ma J, Goldman-Rakic P (2006) Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci 9:534–542
Webb S, Long J, Nelson C (2005) A longitudinal investigation of visual event-related potentials in the first year of life. Dev Sci 8(6):605–616
Wennekers T, Palm G (2000) Cell assemblies, associative memory and temporal structure in brain signals. In: Miller R (ed) Time and the brain: conceptual advances in brain research, vol 2. Harwood Academic Publishers, Switzerland, pp 251–274
Wessberg J, Stambaugh C, Kralik J, Beck P, Laubach M, Chapin J, Kim J, Biggs S, Srinivasan M, Nicolelis M (2000) Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 408:361–365
Wichert A (2001) Pictorial reasoning with cell assemblies. Connect Sci 13(1):1–42
Wickelgren W (1999) Webs, cell assemblies, and chunking in neural nets. Can J Exp Psychol 53(1):118–131
Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G (1995) Sharp wave-associated high-frequency oscillation (200hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15(1):30–46
Yoshida M, Hasselmo M (2009) Persistent firing supported by an intrinsic cellular mechanism in a component of the head direction system. J Neurosci 29(15):4945–4952
Yuste R, MacLean J, Smith J, Lansner A (2005) The cortex as a central pattern generator. Nat Rev Neurosci 6:477–483
Zelano C, Montag J, Khan R, Sobel N (2009) A specialized odor memory buffer in primary olfactory cortex. PLOS One 4(3):e4965
Zhou Y, Fuster J (1996) Mnemonic neuronal activity in somatosensory cortex. PNAS 93:10533–10537
Zucker R, Regehr W (2002) Short-term synaptic plasticity. Ann Rev Physiol 64:355–405
Acknowledgments
This work was supported by EPSRC Grant EP/D059720. Thanks to Ray Adams, Eric Chown, Dan Diaper, Kailash Nadh and Pieter DeVries for comments on this paper, and Mark Dubin for the use of his Brodmann figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huyck, C.R., Passmore, P.J. A review of cell assemblies. Biol Cybern 107, 263–288 (2013). https://doi.org/10.1007/s00422-013-0555-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-013-0555-5