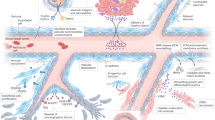
Abstract
The wall of myocardial terminal vessels, consisting of a continuous endothelial tube with an adventitial coat of pericytes in their extracellular matrix, constitutes a remarkably tight barrier to solute transport between the blood and the parenchyma. This constructional principle of precapillary arterioles, capillaries and postcapillary venules extends both up- and downstream into the arterial and venous limbs, where the original microvessel tube widens and becomes the innermost layer—the intima—of all the larger coronary vessels. In the myocardium’s smallest functional units and in the intima of the coronaries, the pericytes play key roles by virtue of both their central histological localization and their physiological functions. Recognition and integration of these properties has led to new pathogenetic models for diverse heart diseases and suggests that current therapeutic concepts need to be revised.




Similar content being viewed by others
Abbreviations
- AP:
-
Alkaline phosphatase
- ECM:
-
Extracellular matrix
- IL:
-
Interleukin
- NG2:
-
Neuronal/glial chondroitin sulphate proteoglycan
- NGF:
-
Nerve growth factor
- NO:
-
Nitrogen monoxide
- PAI-1:
-
Plasminogen activator inhibitor
- PDGF:
-
Platelet derived growth factor
- PDGFR-β:
-
Platelet derived growth factor receptor β
- SEM:
-
Scanning electron micrograph
- SMC:
-
Smooth muscle cells
- TGF-β:
-
Transforming growth factor
- TEM:
-
Transmission electron microscopy
References
Andreeva ER, Pugach IM, Gordon D, Orekhov AN (1998) Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue & cell 30(1):127–135
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21(2):193–215. doi:10.1016/j.devcel.2011.07.001
Ausoni S, Sartore S (2009) The cardiovascular unit as a dynamic player in disease and regeneration. Trends Mol Med 15(12):543–552. doi:10.1016/j.molmed.2009.10.002
Bagher P, Segal SS (2011) Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202(3):271–284. doi:10.1111/j.1748-1716.2010.02244.x
Becerra J, Santos-Ruiz L, Andrades JA, Mari-Beffa M (2011) The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev 7(2):248–255. doi:10.1007/s12015-010-9195-5
Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P (2011) The CNS microvascular pericyte: Pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8(1):8. doi:10.1186/2045-8118-8-8
Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91(4):1800–1809. doi:10.1172/JCI116391
Canfield AE, Doherty MJ, Wood AC, Farrington C, Ashton B, Begum N, Harvey B, Poole A, Grant ME, Boot-Handford RP (2000) Role of pericytes in vascular calcification: A review. Z Kardiol 89(Suppl 2):20–27
Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Peault B (2009) Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev 20(5–6):429–434. doi:10.1016/j.cytogfr.2009.10.014
Corselli M, Chen CW, Crisan M, Lazzari L, Peault B (2010) Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 30(6):1104–1109. doi:10.1161/ATVBAHA.109.191643
del Zoppo GJ (2010) The neurovascular unit, matrix proteases, and innate inflammation. Annals of the New York Academy of Sciences 1207:46–49. doi:10.1111/j.1749-6632.2010.05760.x
Dore-Duffy P (2008) Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 14(16):1581–1593
Eberth CJ (1871) Handbuch der Lehre von den Geweben des Menschen und der Tiere, Vol 1. Leipzig
Edgley AJ, Krum H, Kelly DJ (2012) Targeting fibrosis for the treatment of heart failure: A role for transforming growth factor-beta. Cardiovasc Ther 30(1):e30–40. doi:10.1111/j.1755-5922.2010.00228.x
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R (2010) Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30(7):1282–1292. doi:10.1161/ATVBAHA.108.179739
Gharaibeh B, Lavasani M, Cummins JH, Huard J (2011) Terminal differentiation is not a major determinant for the success of stem cell therapy—cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther 2(4):31. doi:10.1186/scrt72
Gherghiceanu M, Popescu LM (2012) Cardiac telocytes—their junctions and functional implications. Cell Tissue Res 348(2):265–279. doi:10.1007/s00441-012-1333-8
Ghosh AK, Vaughan DE (2012) PAI-1 in tissue fibrosis. J Cell Physiol 227(2):493–507. doi:10.1002/jcp.22783
Han DG (2010) The innateness of coronary artery: vasa vasorum. Med Hypotheses 74(3):443–444. doi:10.1016/j.mehy.2009.10.001
Juchem G, Weiss DR, Gansera B, Kemkes BM, Mueller-Hoecker J, Nees S (2010) Pericytes in the macrovascular intima: possible physiological and pathogenetic impact. Am J Physiol Heart Circ Physiol 298(3):H754–770. doi:10.1152/ajpheart.00343.2009
Juchem G, Weiss DR, Knott M, Senftl A, Forch S, Fischlein T, Kreuzer E, Reichart B, Laufer S, Nees S (2012) Regulation of coronary venular barrier function by blood borne inflammatory mediators and pharmacological tools: insights from novel microvascular wall models. Am J Physiol Heart Circ Physiol 302(3):H567–581. doi:10.1152/ajpheart.00360.2011
Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM (2012) Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol 303(3):H256–270. doi:10.1152/ajpheart.00221.2012
Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P (2011) Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res 109(8):894–906. doi:10.1161/CIRCRESAHA.111.251546
Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ (2006) Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab 26(2):209–217. doi:10.1038/sj.jcbfm.9600181
Kutcher ME, Herman IM (2009) The pericyte: cellular regulator of microvascular blood flow. Microvasc Res 77(3):235–246. doi:10.1016/j.mvr.2009.01.007
McCullough PA, Olobatoke A, Vanhecke TE (2011) Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med 12(4):200–210. doi:10.3909/ricm0624
Muller JM, Davis MJ, Chilian WM (1996) Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res 32(4):668–678
Murakami M, Simons M (2009) Regulation of vascular integrity. J Mol Med (Berl) 87(6):571–582. doi:10.1007/s00109-009-0463-2
Murshed M, McKee MD (2010) Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr Opin Nephrol Hypertens 19(4):359–365. doi:10.1097/MNH.0b013e3283393a2b
Nees S, Herzog V, Becker BF, Bock M, Des Rosiers C, Gerlach E (1985) The coronary endothelium: a highly active metabolic barrier for adenosine. Basic Res Cardiol 80(5):515–529
Nees S, Juchem G, Eberhorn N, Thallmair M, Forch S, Knott M, Senftl A, Fischlein T, Reichart B, Weiss DR (2012) Wall structures of myocardial precapillary arterioles and postcapillary venules reexamined and reconstructed in vitro for studies on barrier functions. Am J Physiol Heart Circ Physiol 302(1):H51–68. doi:10.1152/ajpheart.00358.2011
Nees S, Juchem G, Weiss DR, Partsch H (2012) Pathogenesis and therapy of chronic venous disease: new insights into structure and function of the leg venous system. Phlebologie 41:246–257
Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G (2012) Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol 302(1):H69–84. doi:10.1152/ajpheart.00359.2011
Osterud B, Bjorklid E (2006) Sources of tissue factor. Semin Thromb Hemost 32(1):11–23. doi:10.1055/s-2006-933336
Pober JS, Tellides G (2012) Participation of blood vessel cells in human adaptive immune responses. Trends Immunol 33(1):49–57. doi:10.1016/j.it.2011.09.006
Popescu LM, Faussone-Pellegrini MS (2010) TELOCYTES—a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. Journal of cellular and molecular medicine 14(4):729–740. doi:10.1111/j.1582-4934.2010.01059.x
Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, Pierson RN 3rd, Griffith BP (2006) Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. The Journal of thoracic and cardiovascular surgery 131(1):122–130. doi:10.1016/j.jtcvs.2005.08.058
Proebstl D, Voisin MB, Woodfin A, Whiteford J, D'Acquisto F, Jones GE, Rowe D, Nourshargh S (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209(6):1219–1234. doi:10.1084/jem.20111622
Ribatti D, Crivellato E (2012) “Sprouting angiogenesis”, a reappraisal. Dev Biol 372(2):157–165. doi:10.1016/j.ydbio.2012.09.018
Rusu MC, Pop F, Hostiuc S, Curca GC, Jianu AM, Paduraru D (2012) Telocytes form networks in normal cardiac tissues. Histol Histopathol 27(6):807–816
Sa-Pereira I, Brites D, Brito MA (2012) Neurovascular unit: a focus on pericytes. Mol Neurobiol 45(2):327–347. doi:10.1007/s12035-012-8244-2
Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL (2012) ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J Exp Med 209(12):2291–2305. doi:10.1084/jem.20111749
Socha MJ, Behringer EJ, Segal SS (2012) Calcium and electrical signalling along endothelium of the resistance vasculature. Basic & clinical pharmacology & toxicology 110(1):80–86. doi:10.1111/j.1742-7843.2011.00798.x
Tijsen AJ, Pinto YM, Creemers EE (2012) Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res 93(4):573–582. doi:10.1093/cvr/cvr344
Vanhoutte PM, Shimokawa H, Tang EH, Feletou M (2009) Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196(2):193–222. doi:10.1111/j.1748-1716.2009.01964.x
Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K (2012) Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One 7(9):e45499. doi:10.1371/journal.pone.0045499
Weiss DR, Juchem G, Kemkes BM, Gansera B, Nees S (2009) Extensive deendothelialization and thrombogenicity in routinely prepared vein grafts for coronary bypass operations: facts and remedy. Int J Clin Exp Med 2(2):95–113
Whyte MP (2010) Physiological role of alkaline phosphatase explored in hypophosphatasia. Annals of the New York Academy of Sciences 1192:190–200. doi:10.1111/j.1749-6632.2010.05387.x
Zimmermann KW (1923) Der feinere Bau der Blutcapillaren. Zeitschr f d ges Anat 68:29–109
Acknowledgments
We thank Barbara Nees for excellent pictorial design and photographic work.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Nees, S., Weiss, D.R. & Juchem, G. Focus on cardiac pericytes. Pflugers Arch - Eur J Physiol 465, 779–787 (2013). https://doi.org/10.1007/s00424-013-1240-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1240-1