Abstract
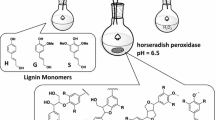
The mechanism of lignin carbohydrate complex formation by addition of polysaccharides on quinone methide (QM) generated during lignin polymerisation was investigated using a model approach. Dehydrogenation polymers (DHPs, lignin model compounds) were synthesized from coniferyl alcohol in the presence of a glucuronoarabinoxylan (GAX) extracted from oat spelts, by Zutropfverfahren (ZT) and Zulaufverfahren (ZL) methods. The methods ZT and ZL differed in their distribution of QM over the reaction period but generated roughly the same QM amount. Steric exclusion chromatography of the ZT and ZL reaction products showed that only the ZT reaction produced high molar mass compounds. Covalent linkages in the ZT reaction involving ether bonds between GAX moiety and α carbon of the lignin monomer were confirmed by 13C NMR and xylanase-based fractionation. The underlying phenomena were further investigated by examining the interactions between GAX and DHP in sorption experiments. GAX and DHPs were shown to interact to form hydrophobic aggregates. In the ZT process, slow addition permitted polymer reorganisation which led to dehydration around the lignin-like growing chains thereby limiting the addition of water on the quinone methide formed during polymerisation and thus favoured lignin–carbohydrate complex (LCC) formation.









Similar content being viewed by others
Abbreviations
- CA:
-
Coniferyl alcohol
- CPMAS:
-
Cross polarization magic angle spinning
- DHP:
-
Dehydrogenation polymer
- DMSO:
-
Dimethyl sulfoxide
- GAX:
-
Glucuronoarabinoxylan
- HPAEC:
-
High-pressure anion exchange chromatography
- LCC:
-
Lignin–carbohydrate complex
- LiBr:
-
Lithium bromide
- NMR:
-
Nuclear magnetic resonance
- QM:
-
Quinone methide
- SEC:
-
Steric-exclusion chromatography
- ZL:
-
Zulaufverfahren
- ZT:
-
Zutropfverfahren
References
Björkman A (1957) Studies on finely divided wood. Part 3. Extraction of lignin–carbohydrate complexes with neutral solvents. Svensk Papperstidning 60:158–159
Cathala B, Monties B (2001) Influence of pectins on the solubility and the molar mass distribution of dehydrogenative polymers (DHPs, lignin model compounds). Int J Biol Macromol 29:45–51
Cathala B, Saake B, Faix O, Monties B (1998) Evaluation of the reproducibility of the synthesis of dehydrogenation polymer models of lignin. Pol Deg Stab 59:65–69
Cathala B, Saake B, Faix O, Monties B (2003) Association behaviour of lignins and lignin model compounds studied by multidetector size-exclusion chromatography. J Chrom A 1020:229–239
Cathala B, Rondeau-Mouro C, Lairez D, Bedos-belval B, Durand H, Gorrichon L, Touzel JP, Chabbert B, Monties B (2005) Models systems for the understanding of lignified plant cell wall formation. Plant Biosyst 139:93–97
Fry SC (1986) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37:165–186
Grabber JH, Ralph J, Hatfield RD (2000) Cross-linking of maize walls by ferulate dimerization and incorporation into lignin. J Agric Food Chem 48:6106–6113
Grabber J, Ralph J, Hatfield RD (2002) Model studies of ferulate-coniferyl-alcohol cross-product formation in primary maize walls: implications for lignification in grasses. J Agric Food Chem 50:6008–6016
Hayashi K, Mardsen M, Delmar D (1987) Pea xyloglucan and cellulose: xyloglucan–cellulose interactions in vitro and in vivo. Plant Physiol 83:384–389
Hemmingson J, Leary G (1975) The chemistry of reactive lignin intermediates. Part II. Addition reactions of vinyl-substituted quinone methides in aqueous solution. J Chem Soc Perkin II:1584–1587
Higuchi T, Ogino K, Tanahashi M (1971) Effect of polysaccharides on dehydropolymerization of coniferyl alcohol. Wood Res 51:1–11
Inomana F, Takabe K, Saiki H (1992) Cell wall formation of conifer tracheid as revealed by rapid-freeze and substitution method. J Electron Microsc 41:369–374
Kalyanasundaram K, Thomas JK (1997) Environmental effects on vibronic band intensities in pyren monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Koshijima T, Taniguchi T, Tanaka R (1972) Lignin carbohydrate complex. Holzforschung 26:211–218
Kuhlmann KF, Grant DM (1968) The nuclear overhauser enhancement of the carbon-13 magnetic resonnance spectrum of formic acid. J Am Chem Soc 90:7355–7357
Koshijima T, Takahashi N (1988) Molecular properties of lignin–carbohydrate complexes from beech (Fagus crenata) and pine (Pinus densiflora) woods. Wood Sci Technol 22:177–189
Lairez D, Cathala B, Monties B, Bedos-Belval F, Duran D, Gorrichon L (2005) On the first steps of lignification: aggregation during coniferyl alcohol polymerisation in pectin solution. Biomacromolecules 6:763–774
Leary G (1972) The chemistry of reactive lignin intermediates. Part I. Transients in coniferyl alcohol photolysis. J Chem Soc Perkin II 5:640–642
Ludley FH, Ralph J (1996) Improved preparation of coniferyl and sinapyl alcohols. J Agric Food Chem 44:2942–2943
Ohnishi J, Watanabe N, Koshijima T (1992) Synthesis of dehydrogenation polymer–polyoside complexes by peroxidase. Phytochemistry 31:1185–1190
Parkas J, Paulsson M, Westermark U, Terashima N (2001) Solid state NMR analysis of β-13C-enriched lignocellulosic material during light-induced yellowing. Holzforschnung 55:276–282
Porsch B, Sundelöf L (1994) Size exclusion chromatography and dynamic light scattering of dextrans in water: explanation of ion-exclusion behaviour. J Chrom A 669:21–30
Ralph J, Marita JM, Ralph SA, Hatfield RD, Lu F, Ede R, Peng J, Quideau S, Helm RF, Grabber JH, Kim H, Jimenez-Monteon G, Zhang Y, Jung H-JG, Landucci L, MacKay JJ, Sederoff RR, Chapple C, Boudet AM (1999) Solution-state NMR of lignins. In: Argyropoulos DS, Rials T (eds) Advances in lignocellulosics characterization. TAPPI, Atlanta, pp 55–108
Saake B, Kruse T, Puls J (2001) Investigation on molar mass, solubility and enzymatic fragmentation of xylans by multi-detected SEC chromatography. Biores Technol 80:195–204
Salmen L, Olsson AM (1998) Interaction between hemicelluloses, lignin and cellulose: structure-property relationships. J Pulp Pap Sci 24:99–103
Shigematsu M, Goto A, Yoshida S, Tanahashi M, Shinoda Y (1994a) Hydrophobic regions of hemicellulose estimated by fluorescent probe method. Mokuzai Gakkaishi 11:1214–1218
Shigematsu M, Goto A, Yoshida S, Tanashi M, Shinoda Y (1994b) Affinities of monolignols and saccharides determined by the solubility method. Mokuzai Gakkaishi 40:321–327
Sipilä J (1990) On the reactions of quinone methide intermediates during lignin biosynthesis: a study with models compounds. Ph.D thesis, Department of chemistry, University of Helsinki
Sipilä J, Brunow G (1991) On the mechanism of formation of non-cyclic benzyl ether during lignin biosynthesis. Part 4. The reactions of a β-O-4 type quinone methide with carboxylic acids in the presence of phenol. The formation and stability of benzyl esters between lignin and carbohydrate. Holzforschung 45:9–14
Takahashi N. Koshijima T (1987) Properties of enzyme-unhydrolyzable residue of lignin–carbohydrate complexes isolated from Beech Wood. Wood Res 74:1–11
Takahashi N, Koshijima T (1988) Ester linkages between lignin and glucuronoxylan in a lignin–carbohydrate complex from beech (Fagus crenata) wood. Wood Sci Technol 22:231–241
Takahashi N, Azuma J, Koshijima T (1982) Fractionation of lignin–carbohydrate complexes by hydrophobic-interaction chromatography. Carbohydr Res 107:161–168
Tanaka K, Nakatsubo F, Higuchi T (1976) Reactions of guaiacylglycerol-β-guaiacyl ether with several sugars I. Reactions of quinone methide with d-glucuronic acid. Mokuzai Gakkaishi 22:589–590
Terashima N (2001) Possible approaches for studying three dimensional structure of lignin. In: Moroshi N, Komamine A (eds) Molecular breeding of woody plants. Elsevier, Amsterdam, pp 257–261
Terashima N, Fukushima K, He L-F, Takabe K (1993) Comprehensive model of the lignified plant cell wall. In: Jung HG, Buxton DR, Hatfield RD, Ralph J (eds) Forage cell wall structure and digestibility. Amer Soc Agronomy, Madison, pp 247–270
Terashima N, Ralph SA, Landucci LL (1996) New facile syntheses of monolignols glucosides; p-glucocoumaryl alcohol coniferin and syringin. Holzforschung 50:151–155
Terashima N, Hafren J, Westermark U, Vanderhart DL (2002) Non destructive analysis of lignin by NMR spectroscopy of specifically 13C-enriched lignins. Holzforschung 56:43–50
Toikka M, Sipila J, Teleman A, Brunow G (1998) Lignin–carbohydrate model compounds. Formation of lignin methyl arabinoside and lignin methyl galactoside benzyl ethers via quinone methide intermediates. J Chem Soc Perkin Trans 1:3813–3818
Vincken JP, Keizer A, Beldman G, Voragen G (1995) Fractionnation of xyloglucans fragments and their interactions with cellulose. Plant Physiol 108:1579–1585
Winnik F, Regismond ST (1996) Fluorescence methods in the study of the interactions of surfactants polymers. Colloids Surf A Physicochem Eng Asp 118:1–39
Winter H, Barakat A, Cathala B, Saake B (2006) Preparation of arabinoxylan and its sorption on bacterial cellulose during cultivation. In: Fisher K, Heinze T (eds) Makromolecular symposium series: hemicelluloses. Wiley, Weinheim, pp 85–92
Xi Y, Yasuda S, Wu H, Liu H (2000) Analysis of the structure of lignin–carbohydrate complexes by the specific 13C tracer method. J Wood Sci 46:130–136
Zykwinska AW, Ralet MCJ, Garnier CD, Thibault JFJ (2005) Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol 139:397–407
Acknowledgments
Access to the NMR facilities of the BIBS platform (Biopolymers, Interactions, Structural Biology) of INRA-Nantes was greatly appreciated by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barakat, A., Winter, H., Rondeau-Mouro, C. et al. Studies of xylan interactions and cross-linking to synthetic lignins formed by bulk and end-wise polymerization: a model study of lignin carbohydrate complex formation. Planta 226, 267–281 (2007). https://doi.org/10.1007/s00425-007-0479-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0479-1