Abstract
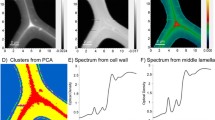
Acylesterification is one of the common modifications of cell wall non-cellulosic polysaccharides and/or lignin primarily in monocot plants. We analyzed the cell-wall acylesters of black cottonwood (Populus trichocarpa Torr. & Gray) with liquid chromatography–mass spectrometry (LC–MS), Fourier transform-infrared (FT-IR) microspectroscopy, and synchrotron infrared (IR) imaging facility. The results revealed that the cell wall of dicotyledonous poplar, as the walls of many monocot grasses, contains a considerable amount of acylesters, primarily acetyl and p-hydroxycinnamoyl molecules. The “wall-bound” acetate and phenolics display a distinct tissue specific-, bending stress responsible- and developmental-accumulation pattern. The “wall-bound” p-coumarate predominantly accumulated in young leaves and decreased in mature leaves, whereas acetate and ferulate mostly amassed in the cell wall of stems. Along the development of stem, the level of the “wall-bound” ferulate gradually increased, while the basal level of p-coumarate further decreased. Induction of tension wood decreased the accumulation of the “wall-bound” phenolics while the level of acetate remained constant. Synchrotron IR-mediated chemical compositional imaging revealed a close spatial distribution of acylesters with cell wall polysaccharides in poplar stem. These results indicate that different “wall-bound” acylesters play distinct roles in poplar cell wall structural construction and/or metabolism of cell wall matrix components.





Similar content being viewed by others
Abbreviations
- FT-IR:
-
Fourier-transform infrared
- IR:
-
Infrared
- LC–MS:
-
Liquid chromatography–mass spectrometry
- GalA:
-
Galacturonic acid
References
Andersson-Gunneras S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, Nilsson P, Henrissat B, Moritz T, Sundberg B (2006) Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J 45:144–165
Aspeborg H, Schrader J, Coutinho PM, Stam M, Kallas A, Djerbi S, Nilsson P, Denman S, Amini B, Sterky F, Master E, Sandberg G, Mellerowicz E, Sundberg B, Henrissat B, Teeri TT (2005) Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiol 137:983–997
Biely P, MacKenzie CR, Puls J, Schneider H (1986) Cooperativity of esterases and xylanases in the enzymatic degradation of acetyl xylan. Biotechnology 4:731–733
Bunzel M, Ralph J, Lu F, Hatfield RD, Steinhart H (2004) Lignins and ferulate-coniferyl alcohol cross-coupling products in cereal grains. J Agric Food Chem 52:6496–6502
Carpita NC, McCann MC (2000) The cell wall. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 52–108
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1250–1318
Franke R, Hemm MR, Denault JW, Ruegger MO, Humphreys JM, Chapple C (2002) Changes in secondary metabolism and deposition of an unusual lignin in the ref8 mutant of Arabidopsis. Plant J 30:47–59
Fry SC (1986) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol Plant Mol Biol 37:165–186
Fry SC, Miller JC (1989) Toward a working model of the growing plant cell wall. Phenolic cross-linking reactions in the primary cell walls of dicotyledons. In: Lewis NG, Paice MG (eds) Plant cell wall polymers, biogenesis and biodegradation, vol 399. American Chemical Society, Washington, DC, pp 33–46
Grabber JH, Ralph J, Lapierre C, Barrière Y (2004) Genetic and molecular basis of grass cell-wall degradability. I. Lignin–cell wall matrix interactions. C R Biol 327:455–465
Hatfield RD, Ralph J, Grabber JH (1999) Cell wall cross-linking by ferulates and diferulates in grasses. J Sci Food Agric 79:403–407
Iiyama K, Lam TB-T, Stone BA (1994) Covalent cross-links in the cell wall. Plant Physiol 104:315–320
Ishii T (1997a) Structure and functions of feruloylated polysaccharides. Plant Sci 127:111–127
Ishii T (1997b) O-acetylated oligosaccharides from pectins of potato tuber cell walls. Plant Physiol 113:1265–1272
Jacobs A, Lundqvist J, Stalbrand H, Tjerneld F, Dahlmana O (2002) Characterization of water-soluble hemicelluloses from spruce and aspen employing SEC/MALDI mass spectroscopy. Carbohydr Res 337:711–717
Kacuráková M, Wellner N, Ebringerová A, Hromádková Z, Wilson RH, Belton PS (1999) Characterization of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocoll 13:35–41
Kacuráková CP, Sasinková V, Wellner EA (2000) FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym 43:195–203
Kato Y, Nevins DJ (1984) Enzymic dissociation of zea shoot cell wall polysaccharides: IV. Dissociation of glucuronoarabinoxylan by purified endo-(1 → 4)-β-xylanase from Bacillus subtilis. Plant Physiol 75:759–765
Kauppinen S, Christgau S, Kofod LV, Halkier T, Dörreich K, Dalbøge H (1995) Molecular cloning and characterization of a rhamnogalacturonan acetylesterase from Aspergillus aculeatus. J Biol Chem 270:27172–27178
Lam TBT, Kadoya K, Iiyama K (2001) Bonding of hydroxycinnamic acids to lignin: ferulic and p-coumaric acids are predominantly linked at the benzyl position of lignin, not the β-position, in grass cell walls. Phytochemistry 57:987–992
Liners F, Gaspar T, Van Cutsem P (1994) Acetyl- and methyl-esterification of pectins of friable and compact sugar-beet calli: consequences for intercellular adhesion. Planta 192:545–556
Lu FC, Ralph J (1999) Detection and determination of p-coumaroylated units in lignins. J Agric Food Chem 47:1988–1992
Lu FC, Ralph J (2002) Preliminary evidence for sinapyl acetate as a lignin monomer in kenaf. Chem Commun (Camb), pp 90–91
Marchessault RH, Liang CY (1962) The infrared spectra of crystalline polysaccharides. VIII. Xylans. J Polym Sci 59:357–378
McCann MC, Bush M, Milioni D, Sado P, Stacey NJ, Catchpole G, Defernez M, Carpita NC, Hofte H, Ulvskov P, Wilson RH, Roberts K (2001) Approaches to understanding the functional architecture of the plant cell wall. Phytochemistry 57:811–821
Miller LM, Dumas P (2006) Chemical imaging of biological tissue with synchrotron infrared light. Biochim Biophys Acta 1758:846–857
Mouille G, Robin S, Lecomte M, Pagant S, Hofte H (2003) Classification and identification of Arabidopsis cell wall mutants using Fourier-transform infrared (FT-IR) microspectroscopy. Plant J 35:393–404
Pauly M, Anderson LN, Kauppinen S, Kofod LV, York WS, Albersheim P, Darvill AG (1999) A xyloglucan-specific endo-β-1,4-glucanase from Aspergillus aculeatus: expression cloning in yeast, purification and characterization of the recombinant enzyme. Glycobiology 9:93–100
Philippe S, Robert P, Barron C, Saulnier L, Guillon F (2006) Deposition of cell wall polysaccharides in wheat endosperm during grain development: Fourier transform-infrared microspectroscopy study. J Agric Food Chem 54:2303–2308
Piber M, Koehler P (2005) Identification of dehydro-ferulic acid-tyrosine in rye and wheat: evidence for a covalent cross-link between arabinoxylans and proteins. J Agric Food Chem 53:5276–5284
Pippen EL, McCready RM, Owens HS (1950) Gelation properties of partially acetylated pectins. J Am Chem Soc 72:813–816
Ralph J, Bunzel M, Marita JM, Hatfield RD, Lu F, Kim H, Schatz PF, Grabber JH, Steinhart H (2004) Peroxidase dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem Rev 3:79–96
Renard CC, Jarvis MC (1999) Acetylation and methylation of homogalacturonans 1: optimisation of the reaction and characterization of the products. Carbohydr Polym 39:201–207
Robert P, Marquis ML, Barron C, Guillon F, Saulnier L (2005) FT-IR investigation of cell wall polysaccharides from cereal grains. Arabinoxylan infrared assignment. J Agric Food Chem 53:7014–7018
Rombouts FM, Thibault JF (1986) Enzymatic and chemical degradation and the fine structure of pectins from sugar-beet pulp. Carbohydr Res 154:189–203
Schols HA, Voragen AG (1994) Occurrence of pectic hairy regions in various plant cell wall materials and their degradability by rhamnogalacturonase. Carbohydr Res 256:83–95
Sederoff RR, MacKay JJ, Ralph J, Hatfield RD (1999) Unexpected variation in lignin. Curr Opin Plant Biol 2:145–152
Teleman A, Lundqvist J, Tjerneld F, Stålbrand H, Dahlman O (2000) Characterization of acetylated 4-O-methylglucuronoxylan isolated from aspen employing 1H and 13C NMR spectroscopy. Carbohydr Res 329:807–815
Teleman A, Nordström M, Tenkanen M, Jacobs A, Dahlman O (2003) Isolation and characterization of O-acetylated glucomannans from aspen and birch wood. Carbohydr Res 338:525–534
Tenkanen M (1998) Action of Trichoderma reesei and Aspergillus oryzae esterases in the deacetylation of hemicelluloses. Biotechnol Appl Biochem Pt 1:19–24
Timell TE (1964) Wood hemicelluloses. Adv Carbohydr Chem 19:247–302
Timell TE (1969) The chemical composition of tension wood. Sven Papperstidn 72:173–181
Tuskan GA et al (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604
Voragen AGJ, Pilnik W, Thibault J-F, Axelos MAV, Renard MGC (1998) Pectins. In: Dekker M (ed) Food polysaccharides and their applications. Academic Press, New York, pp 287–339
Wende G, Fry SC (1997a) 2-O-β-D-Xylopyranosyl-(5-O-feruloyl)-L-arabinose, a widespread component of grass cell walls. Phytochemistry 44:1019–1030
Wende G, Fry FC (1997b) O-feruloylated, O-acetylated oligosaccharides as side-chains of grass xylans. Phytochemistry 44:1011–1018
Williamson G, Faulds CB, Matthew JA, Archer DB, Morris VJ, Brownsey GJ, Ridout MJ (1990) Gelation of sugarbeet and citrus pectins using enzymes extracted from orange peel. Carbohydr Polym 13:387–397
Acknowledgments
The authors would like to thank Dr. Gray Tuskan in Oak Ridge National Laboratory for providing additional P. trichocarpa plantlets. This work was supported by the DOE-USDA joint Plant Feedstock Genomics Program (Project No. Bo-135) and the Laboratory Directed Research and Development Program (LDRD-07-047) of Brookhaven National Laboratory under contract with Department of Energy to Chang-Jun Liu. Use of the National Synchrotron Light source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gou, JY., Park, S., Yu, XH. et al. Compositional characterization and imaging of “wall-bound” acylesters of Populus trichocarpa reveal differential accumulation of acyl molecules in normal and reactive woods. Planta 229, 15–24 (2008). https://doi.org/10.1007/s00425-008-0799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0799-9