Abstract
Down syndrome (DS) is one of the most common chromosomal abnormalities. Because of medical advances and improvements in overall medical care, the median survival of individuals with DS has increased considerably. This longer life expectancy requires giving the necessary care to the individual with DS over their total longer lifespan. DS medical guidelines are designed for the optimal care of the child in whom a diagnosis of DS has been confirmed. We present an overview of the most important issues related to children with DS based on the most relevant literature currently available.
Similar content being viewed by others
Introduction
Down syndrome (DS) is the most common chromosomal malformation in newborns. In Europe, DS accounts for 8% of all registered cases of congenital anomalies. Throughout the world, the overall prevalence of DS is 10 per 10,000 live births, although in recent years this figure has been increasing. To a large extent, the prevalence of DS depends on several socio-cultural variables. In countries where abortion is illegal such as Ireland and the United Arab Emirates, its prevalence is higher. Conversely, in France, DS prevalence is low, and this is probably due to a high percentage of DS pregnancy terminations [6, 21, 33]. In The Netherlands, the most recent measure of DS prevalence was 16 per 10,000 live births [33]. In the United Kingdom, the prevalence of pregnancies affected by DS has increased significantly, but there has been no overall change in the live birth prevalence of DS. Increasing maternal age and improved survival rates for infants with Down syndrome have outweighed the effects of prenatal diagnosis followed by the termination of pregnancy and a declining general birth rate [6, 14, 24, 33, 36].
DS is characterized by several dysmorphic features and delayed psychomotor development. Children with DS also have an increased risk of concomitant congenital defects and organic disorders such as congenital heart and gastrointestinal defects, celiac disease and hypothyroidism [21]. The median age at death of individuals with DS has risen significantly in the US, from 25 years in 1983 to 49 years in 1997. Congenital heart defects (CHD) and respiratory infections are the most frequently reported medical disorders on death certificates for individuals with DS [38]. Standardized mortality odds ratios (SMORs) in DS were low for malignancies except for leukaemia and testicular cancer, which were seen more often in individuals with DS [21, 39]. Recent decades have seen a substantial increase in the life expectancy of children with DS. In The Netherlands, the infant mortality rate in children with DS dropped from 7.07% in 1992 to 4% in 2003 (this is in contrast with the 0.48% infant mortality of the reference population in The Netherlands in 2003) [33]. The fall in DS mortality was mainly related to the successful early surgical treatment of CHD and to the improved treatment of congenital anomalies of the gastrointestinal tract [33]. The life expectancy of children with DS is primarily dependent on the risk of mortality in the first year of life. While modern medical care has reduced the mortality rate to more acceptable values, both morbidity and mortality could be further reduced. In this respect, respiratory infections and neonatal problems are the most important issues to be solved.
Since children with DS now have an improved life expectancy, the total population of individuals with DS is expected to grow substantially. Preventive health care programmes for these children will contribute to the improvement of their overall outcome and quality of life; therefore, it is very important to keep the medical guidelines updated [11, 21].
Newborn assessment
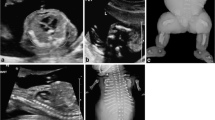
The characteristics of DS and specific clinical signs at birth can guide the decision to perform karyotype testing for the confirmation of a DS diagnosis (Table 1, Figs. 1, 2) [20, 30]. Hypotonia is the most striking characteristic, but others include a “Simian fold”(Fig. 3), drinking problems, signs of a CHD, a congenital defect of the gastrointestinal tract or cataract. It will take several days before the first results of the karyotyping can confirm a clinical suspicion of DS.
Initial postnatal support
The parents or caretakers of a child for whom a diagnosis of DS is being considered or has been confirmed should be informed in a supportive, positive, caring and honest manner [26]. Because parents prefer to receive an accurate and correct diagnosis, the information available to both the paediatrician and the parents should be up-to-date. The conversation must take place with both parents in a quiet setting as soon as the diagnosis of DS is suspected, in the presence of a paediatrician, an obstetrician and the child with DS. The timing of the disclosure of specific DS-related problems must be balanced with respect for the opportunity for parents to welcome their child (Table 2) [19, 26].

Cardiovascular disorders
The prevalence of CHD in neonates with DS is about 44–58% worldwide. Atrioventricular septal defect and ventricular septal defect are the most common forms of CHD, constituting up to 54% for ASD and to 33% for VSD, of all CHDs in children with DS [31, 33]. A normal neonatal examination in children with DS does not exclude a serious CHD. Because of the high incidence of significant CHD in children with Down syndrome, early recognition of CHD is necessary as it can lead to the optimal management of the defect and can sometimes prevent the development of pulmonary hypertension. The surgical correction of significant defects usually takes place at the age of 2–4 months, though it is sometimes performed earlier (e.g. in cases of Tetralogy of Fallot). An elevated incidence (5.2–13.7%) of persistent pulmonary hypertension of the neonate (PPHN) with DS has recently been established, and there should be a specific focus on this condition after birth [35]. Early assessment of the cardiac condition of neonates with DS should always be performed by echocardiography in the first month of life [8, 21, 35].
Vision disorders
Good vision is very important to the development of a child, especially a child with developmental problems such as those associated with DS. More than half of children with DS have ocular abnormalities. In addition to ocular features related to DS such as epicanthal folds, narrowed or slanted palpebral fissures (the mongoloid slant) and Brushfield spots (38–85%)(Fig. 4), these vision disorders include strabismus (20–47%), nystagmus (11–29%), congenital cataract (4–7%), acquired cataract (3–15%), blepharitis (7–41%), refractive errors (43–70%) and glaucoma (0.7%). Keratoconus is rare in childhood but develops later in life in individuals with DS [27, 36].Visual screening is essential for detecting defects that can be treated. An early start is especially important in finding congenital cataracts.
Ear, nose and throat disorders
Hearing impairment and otologic problems are prevalent in children with DS, and these problems correlate substantially with developmental problems. Midface hypoplasia is common in children with DS and consists of abnormalities of the nasopharynx, abnormal Eustachian tube anatomy, abnormal tooth development and agenesis of the teeth. These mid-face problems, together with hypotonia and macroglossia (children with DS have a relatively large tongue compared to the oral cavity), are responsible for chronic middle ear disease and chronic rhinorrhoea.
Allergy does not play an important role as a cause of chronic rhinitis in children with DS [17]. On the other hand, a variety of immune disorders makes them prone to upper airway infections [4]. Even mild hearing loss can influence educational, language and emotional developments, and as a result, it can affect a child’s articulation skills. Regular assessment of the hearing function is very important. An active search for and treatment of chronic ear disease in children with DS, started soon after birth, may improve hearing [15]. Apart from hearing problems, children with DS have delayed speech development [21]. Sleep-disordered breathing in children with Down syndrome is seen in half of the children with DS. The most common causes include macroglossia, glossoptosis, recurrent enlargement of the adenoid tonsils and enlarged lingual tonsils. There is a poor correlation between parental impressions of sleep problems and polysomnography results. Baseline polysomnography must be considered in children with Down syndrome at 3 to 4 years of age [8, 25].

Respiratory disorders
Respiratory problems are responsible for the majority of the morbidity and hospital admissions in children with DS. Respiratory syncytial virus (RSV) is seen more frequently and is associated with a greater risk for hospitalization in children with DS. CHD does not influence the admission rate, but children with CHD had longer lengths of hospital stay [4, 13]. Recurrent wheeze is very common among children with DS (it is found in up to 36%) and is related to previous RSV infection and to other factors such as tracheamalacia [3, 4]. The clinical picture may mimic asthma but is not equivalent to asthma. These respiratory problems can in turn become exacerbated because of the existence of CHD with haemodynamic instability and as a result of hypotonia, both known characteristics of DS. Other causal factors include airway anomalies like tracheolaryngomalacia, pulmonary anatomical changes like pulmonary hypoplasia, and subpleural cysts. Subpleural cysts are common in individuals with DS (up to 36%) but are difficult to detect on plain chest films—CT imaging is needed to detect them [2]. Furthermore, an association with abnormal lung growth and lung hypoplasia is found in children with DS [1]. RSV prophylaxis with human monoclonal antibodies in children with DS with CHD is common, but in a child without CHD, the prophylaxis has to be considered because of their risk of the more frequent and serious infections associated with RSV [3].

Gastrointestinal tract disorders
Congenital defects of the gastrointestinal tract are present in 4–10% of children with DS and play an important role in morbidity during the first year of life. These defects include oesophageal atresia/trachea-oesophageal fistula (0.3–0.8%), pyloric stenosis (0.3%), duodenal stenosis/atresia (1–5%), Hirschsprung disease (1–3%) and anal stenosis/atresia (<1–4%).These defects are more frequent in the DS population, as much as 25–30% of all cases of duodenal defects are in children with DS [9]. Coeliac disease (CD) is another DS-specific disorder and is seen in 5–7% of children with DS, a rate that is ten times higher than in the normal population [38]. Screening for early detection of CD in the DS population, with the aim of starting treatment and preventing complications from untreated CD such as failure to thrive, anaemia, osteoporosis and malignancy, seems to be justified. For CD screening in children with DS, we recommend human leukocyte antibodies (HLA)-DQ2 and HLA-DQ8 typing in the first year of life with buccal swabs, if available, which have the benefit of avoiding the unpleasant collection of blood. Children who have negative results for HLA-DQ2 or DQ8 (approximately 60%) can be excluded from further screening, and parents can be reassured that their child has no risk of CD. The remaining children need to be monitored for CD by using IgA anti-endomysium (EMA) and anti-tissue transglutaminase antibodies (anti-tTGA) beginning at 3 years of age [15, 38].
The prenatal occurrence of an aberrant right subclavian artery (ARSA, arteria lusoria) is substantially increased in patients with DS where it is found in up to 19–36%. ARSA may cause problems with the passage of solid food through the oesophagus and dysphagia. Moreover, impaired oral motor function, gastro-oesophageal reflux or congenital disorders have to be considered as a cause in feeding problems in children with DS [9, 22].
Constipation is a serious problem in children with DS and is mainly a consequence of the hypotonia; however, in serious cases, Hirschprung disease must be excluded [8].
Breastfeeding should be promoted not only because of the psycho-emotional or immunity benefits but particularly because breastfeeding has specific advantages for children with DS in terms of stimulating the development of the oral motor system [33]. However, because of their impaired oral motor function, children with DS can have problems with drinking, swallowing and chewing.
Overweight and overnutrition deserve serious attention in children with DS [21].
Haemato-oncological and immunological disorders
Newborns with DS may have thrombocytopenia (up to 66%) and polycythaemia (up to 33%) [39]. The first must be differentiated from pre-leukaemia while the latter may be symptomatic and may cause hypoglycemia or respiratory problems. Children with DS have an increased risk of developing both acute myeloid as well as lymphoblastic leukaemia. These leukaemias have specific presenting characteristics and underlying biologies. Myeloid leukaemia in children with DS may be preceded by a preleukaemic clone (transient myeloproliferative disorder, TMD), which may disappear spontaneously but may need treatment when symptoms are severe. TMD presents in 10% of children with DS. Twenty percent of children with transient leukaemia subsequently develop myeloid leukaemia, usually with an onset before the age of 5 [39]. Children with DS have lowered T- and B-lymphocyte counts and functions which may explain part of their susceptibility to infections. On the other hand, there is a lower allergy risk in children with DS [4, 17, 18, 23, 39]. There is no specific clinical picture in relation to disturbances of the immune system, which means that screening is not useful.

Endocrine disorders
In neonates with DS, the Gaussian distribution of thyroxin and TSH values are shifted to the left, and there may be a DS-specific thyroid dysregulation. Thyroxin has been given to newborns during the first 24 months, and although short-term follow-up showed some benefit to development and growth, there is no data available on the long-term benefit of treating these children with thyroxin [10, 28, 29]. Therefore, this treatment is not commonly used.
Thyroid disorders have been reported in up to 28–40% of children with DS, and they increase in frequency, up to 54%, as the children age [10, 15, 28]. Thyroid abnormalities in children with DS range from congenital hypothyroidism (1.8–3.6%) to primary hypothyroidism, autoimmune (Hashimoto) thyreoiditis (0.3–1.4%) and compensated hypothyroidism (25.3–32.9%). In addition, hyperthyroidism (Graves’ disease) (0–2%) occurs in children with DS as well. Compensated hypothyroidism or isolated raised thyroid stimulating hormone (IR-TSH) is most frequently present in children with DS and is characterized by mildly elevated TSH with normal or low normal free T4; it often has a self-limiting natural history [10]. These thyroid antibodies are the second most frequently present, and when present, they can cause manifest hypo-or hyperthyroidism within 2 years in almost 30%, but these antibodies are as such not primary related to abnormal thyroid function [10, 15, 28]. Most predictive of the development of hypothyroidism is the presence of both elevated TSH and antibodies, and those children should be tested more frequently than other children with DS [9]. When both are normal in the first decade of life, there is a low probability of hypothyroidism in the second decade [10]. Interestingly, diabetes mellitus develops more frequently (1%) in children with Down syndrome [21, 30].
Children with DS have their own growth pattern and DS-specific growth curves [7].The follow-up of length and weight in children with DS should be part of the regular medical screening and special attention for the weight is warranted because children with DS are prone to overweight. Their lack of feeling of satisfaction and their unlimited food intake, as well as their moderate exercise pattern, need special attention [21].
Orthopaedic disorders
The motoric system of children with DS is characterized by ligamentous laxity, joint hypermobility and hypotonia presenting in a variety of ways [5, 15]. Craniocervical instability has been reported in 8% to 63% of children with DS; atlanto-axial instability (AAI) occurs in 10% to 30%. The majority of cases are asymptomatic with symptomatic disease occurring in 1% to 2%, particularly as the result of an accident [12]. There are limitations regarding both obtaining and interpreting and screening X-rays for AAI, and these are not predictive for injury. The performance of yearly neurologic screening is advisable, as is taking extra care when intubation is necessary. Sport activities, including somersaults, can be part of these patients’ activities as long as there is a good support [5, 12, 21]. Individuals with DS have been described as having a specific gait, with external rotation of the hips, knees in flexion and valgus, and externally rotated tibias. In childhood, pes planovalgus is often seen, and in cases where marked pronation of the foot creates problems with stable ambulation, active support is warranted [21] (Fig. 5).
Acquired hip dislocation occurs in up to 30% of children with DS and needs special attention. Patellofemoral instability is estimated to occur in 10–20% of children with DS; slipped capital femoral epiphysis is seen more often in children with DS and has a poor prognosis [5]. Most of these disorders manifest themselves once children with DS start walking, around 2–3 years of age [5]. An arthropathy similar to juvenile rheumatoid arthritis can develop in children with DS but is rare [5]. The delay in motor development in children with DS is more pronounced than the delay in mental development. Delays in motor development appear to be particularly related to the degree of hypotonia, which negatively influences development and leads to problems in postural control and to typically static and symmetrical movement patterns, compensatory movement strategies, and lack of movement variability. Limitations in the functional activities of 5 to 7-year-old children with Down syndrome seem to be more related to the level of motor ability than to the level of performance of mental ability [32]. Special attention to motor development and counselling by a physiotherapist is advised.
DS directed physiotherapy supports the development of the basic gross motor skills properly by challenging the children with DS and giving them confidence in their own physical abilities by making use of the knowledge of the typically movement patterns of DS, furthermore to support parents to start active play and sports.
Urinary tract disorders
Children with DS have significantly more risk of urinary tract anomalies (UTAs) (3.2%). Symptoms may be masked because voiding disturbances and delayed toilet training are usually interpreted as a consequence of delayed psychomotor development. UTAs such as hydronephrosis, hydroureter, renal agenesis and hypospadias must be considered in children with DS. Routine screening by ultrasound is not yet standard, but paediatricians should not overlook this problem [16].
There are no specific guidelines towards the attitude in delay in daytime and nighttime continence in children with DS; besides the standard treatment, visual instruction is helpful as well as showing them how to do. The advice is to start training at the moment the child can sit properly and understand the items stool, urine and toilet.
Sexual development
In adolescent girls with DS, the onset of puberty is similar to that of other adolescents. In boys with DS, the primary and secondary sexual characteristics and pituitary and testicular hormone concentrations are similar to those in typical adolescents [21]. Females with DS are able to have children, but males with DS have a diminished capacity to reproduce [21]. Education to prevent pregnancies is warranted, specific in children with DS who discuss sexuality open-hearted. In girls, contraception can only be discussed when their mental development enables them to understand the subject.
Furthermore, contraception can be given to prevent pregnancy or when there are problems with the menstrual cycle, for fear of blood or problems with the hygiene.
Unfortunately one must be cautious for sexual abuse in girls with DS.
Dermatologic problems
Dermatologic diseases are often present and are especially troublesome in adolescents [21]. Alopecia areata (2.9–20%), vitiligo (1.9%), seborrhoeic eczema (8–36%), folliculitis (10.3–26%) and syringoma (12.3–39.2%) are more frequently seen in children with DS. Rare but DS-specific problems are elastosis perforans serpiginosa and milia-like idiopathic calcinosis cutis [18, 23]. A previously reported high prevalence of atopic dermatitis (AD) in up to 56.5% of children with DS is probably an overestimation, as more recent studies suggest a lower prevalence of 1.4–3%. This could be the result of new and different diagnostic criteria for AD. This observation also notes a lower allergy risk in children with DS, which is in concordance with the studies on allergic rhinitis [17, 18, 23].
Neuro-behavioural disorders
Most children with DS function in the low range of typical development, and their intelligence quotient decreases in the first decade of life. In adolescence, cognitive function may reach a plateau that persists in adulthood. Mental development shows a deceleration between the ages of 6 months and 2 years [32]. IQ values vary, usually ranging from 35 to 70, indicating mild to moderate mental impairment; severe mental impairment is only occasionally seen in children with DS [8]. Counterproductive behaviour and avoidance tactics can impede learning, and language production is often substantially impaired. Delayed verbal short-term memory and expressive language indicate the need for a special approach to teaching these children to speak (for example, learning to speak by first learning to read) [15, 21]. Furthermore, impaired oral motor function can influence articulation.
Children with DS have more pronounced neuro-behavioural and psychiatric problems, found in 18% to 38%. The most frequent problems are disruptive behaviour disorders, such as attention deficit hyperactivity disorder (6.1%), conduct/oppositional disorder (5.4%) or aggressive behaviour (6.5%), and obsessive–compulsive disorders. More than 25% of adults with DS have a psychiatric disorder, most frequently a major depressive disorder (6.1%) or aggressive behaviour (6.1%) [15, 21]. A diagnosis of autism or autism spectrum disorders in children with DS is found in 7%. This diagnosis is not easily made in children with DS mainly because of the resemblance and overlap of DS-specific behaviours and autism.
Epilepsy is seen in 8% of children with DS, with 40% occurring in infancy and often presenting as infantile spasms. Alzheimer’s disease which is associated with DS appears later in life, not in childhood [21].
Education and school
Early intervention education systems are programmes that can be used from the first months of life and provide tools to stimulate the development of children with DS, especially in the preschool period. Children with DS often begin primary school with extra support; successful outcomes are mainly in the area of social skills as a result of the ability to copy and mirror behaviour. The outcome for adult social independence depends largely on the development of abilities to complete tasks without assistance, the willingness to separate emotionally from parents and access to personal recreational activities [21].
Conclusion and recommendations
Children with DS have several DS-specific morbidities and screening programmes are available to support and educate patients and their families. Although the most frequently occurring morbidities are emphasized, a potential drawback is that a child with DS might have rare DS-specific problems, but children with DS can also have the same problems as their healthy peers. Today children with DS have a better life expectancy, which means that the total population of individuals with DS is expected to grow substantially. There should be a focus on probable changes in long-term DS morbidity. Furthermore, we need to address the quality of this longer life span. Our recommendations for regular screening are shown in Table 3.
Abbreviations
- DS:
-
Down syndrome
- OSAS:
-
Obstructive sleep apnea syndrome
- AAI:
-
Atlanto-axial instability
- RSV:
-
Respiratory syncytial virus
- UTA:
-
Urinary tract anomalies
- AD:
-
Atopic dermatitis
- anti-tTG:
-
Anti-tissue transglutaminase antibodies
- HLA:
-
Human leukocyte antibodies
References
Bertrand P, Navarro H, Caussade S et al (2003) Airway anomalies in children with Down syndrome: endoscopic findings. Ped Pulm 36:137–141
Biko DM, Schwartz M, Anupindi SA, Altes TA (2008) Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 38:280–284
Bloemers BLP, van Furth AM, Weijerman ME et al (2007) Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis—a prospective birth-cohort study. Pediatrics 120:e1076–e1081
Bloemers BLP, van Furth AM, Weijerman ME et al (2010) High incidence of recurrent wheeze in children with Down syndrome with and without previous respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J 29:39–42
Caird MS, Wills BP, Dormans JP (2006) Down syndrome in children: the role of the orthopaedic surgeon. J Am Acad Orthop Surg 14:610–619
Collins VR, Muggli EE, Riley M et al (2008) Is Down syndrome a disappearing birth defect? J Pediatr 152:20–24
Cremers MJ, van der Twee I, Boersma B et al (1996) Growth curves of Dutch children with Down syndrome. J Intellect Disabil Res 40:412–420
Cunnif C, Frias JL, Kaye C et al (2001) Health supervision for children with Down syndrome, American Academy of Pediatrics. Pediatrics 107:442–449
Freeman SB, Torfs CA, Romitti MH et al (2009) Congenital gastrointestinal defects in Down syndrome: a report from the Atlanta and National Down Syndrome Projects. Clin Genet 75:180–184
Gibson PA, Newton RW, Selby K et al (2005) Longitudinal study of thyroid function in Down’s syndrome in the first two decades. Arch Dis Child 09:575–578
Guidelines for basic essential medical surveillance. The Down’s syndrome medical interest group UK and Ireland. Available at: http://www.dsmig.org.uk/publications/guidelines.html Accessed 29 Jan 2010
Hankinson TC, Anderson RC (2010) Craniovertebral junction abnormalities in Down syndrome. Neurosurgery 66:A32–A38
Hilton JM, Fitzgerald DA, Cooper DM (1999) Respiratory morbidity of hospitalized children with Trisomy 21. Paediatr Child Health 35:383–386
Irving C, Basu A, Richmond S et al (2008) Twenty-year trends in prevalence and survival of Down syndrome. Eur J Hum Genet 16:1336–1340
Kishnani PS, Crissman BG (2006) Special issue: current perspectives on Down syndrome: selected medical and social issues. Am J Med Genet C Semin Med Genet 142C:127–205
Kupferman JC, Druschel CM, Kupchik GS (2009) Increased prevalence of renal urinary tract anomalies in children with Down syndrome. Pediatrics 124:e615–e621
Mannan SE, Ejaz Y, Hossain J (2009) Prevalence of positive skin prick test results in children with Down syndrome: a case control study. Ann Allergy Asthma Immunol 102:2005–2009
Madan V, Williams J, Lear JT (2006) Dermatological manifestations of Down’s syndrome. Clin Exp Dermatol 31(5):623–629
Muggli EE, Collins VR, Marraffa C (2009) Going down a different road: first support and information needs of families with a baby with Down syndrome. MJA 190:58–61
Rex AP, Preus M (1982) A diagnostic index for Down syndrome. J Pediatr 6:903–906
Roizen NJ, Patterson D (2003) Down’s syndrome. Lancet 361:1281–1289
Roofthooft MT, van Meer H, Rietman WG et al (2008) Down syndrome and aberrant right subclavian artery. Eur J Pediatr 167:1033–1036
Schepis C, Barone C, Siragusa M et al (2002) An updated survey on skin conditions in Down syndrome. Dermatology 20:234–238
Shin M, Besser LM, Kucik JE et al (2009) Prevalence of Down Syndrome among children and adolescents in 10 regions of the United States. Pediatrics 124:1565–1571
Shott SR, Amin R, Chini B et al (2006) Obstructive sleep apnea should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg 132:432–436
Skotko BG, Capone GT, Kishnani S (2009) Postnatal diagnosis of Down syndrome: synthesis of the evidence on how best to deliver the news. Pediatrics 124:e751–e758
Stephen E, Dickson J, Kindley AD et al (2007) Surveillance of vision and ocular disorders in children with Down syndrome. Dev Med Child Neurol 49:513–515
Unachak K, Tanpaiboon P, Yupada Pongprot Y et al (2008) Thyroid functions in children with Down’s syndrome. J Med Assoc Thai 91:56–61
van Trotsenburg ASP, Vulsma T, van Rozenburg-Marres SLR et al (2005) The Effect of thyroxine treatment started in the neonatal period on developmentand growth of two-year-old Down syndrome children: a randomized clinical trial. J Clin Endocrinol Metab 90:3304–3311
van Wouwe JP, Siderius EJ, Borstlap R et al (2001) Optimal medical care for children with Down syndrome and their parents. Ned Tijdschr Geneeskd 145:1617–1621
Vis JC, Duffels MGJ, Winter MM et al (2009) Down syndrome: a cardiovascular perspective. J Intellect Disabil Res 53:419–425
Volman MJ, Visser JJ, Lensvelt-Mulders GJ (2007) Functional status in 5 to 7-year-old children with Down syndrome in relation to motor ability and performance mental ability. Disabil Rehabil 29:25–31
Weijerman ME, van Furth AM, Vonk Noordegraaf A et al (2008) Prevalence, neonatal characteristics and first-year mortality of Down syndrome: a national study. J Pediatrics 152:15–19
Weijerman ME, van Furth AM, Mooren MD et al (2010) Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. doi:10.1007/s00431-010-1200-0
Wiseman FK, Alford KA, Tybulewicz VL et al (2009) Down syndrome—recent progress and future prospects. Hum Mol Genet 18(R1):75–83
Wong V, Ho D (1997) Ocular abnormalities in Down syndrome: an analysis of 140 Chinese children. Pediatr Neurol 16:311–314
Wouters J, Weijerman ME, van Furth AM et al (2009) Prospective HLA, EMA, and TGA testing celiac disease in children with Down syndrome. J Pediatrics 154:239–242
Yang Q, Rasmussen SA, Friedma JM (2002) Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet 359:1019–1025
Zwaan MC, Reinhardt DR, Hizler J, Vyas P (2008) Acute leukemias in children with Down syndrome. Pediatr Clin North Am 55:53–70
Acknowledgments
The authors would like to thank Roel Borstlap, former paediatrician of the Assen Down clinic, for his constructive remarks. The authors were pleased to use the photos of the children with Down syndrome. Parental permission was obtained to publish the photos of the children, photography by www.fluitekruidje.nl.
There are no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Weijerman, M.E., de Winter, J.P. Clinical practice. Eur J Pediatr 169, 1445–1452 (2010). https://doi.org/10.1007/s00431-010-1253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-010-1253-0