Abstract
One of the promising approaches in mucosal immunization relies on live recombinant vaccine carriers. In this study, we used a six-extracellular protease-deficient Bacillus subtilis strain WB600 to express Schistosoma japonicum 26 kDa glutathione S-transferase (GST). Western blot, immunofluorescence, and flow cytometry analyses were used to identify SjGST expression on spore surface. SjGST recombinant spores were used for oral vaccination in mice and were shown to generate mucosal and systemic response. Both SjGST-specific secretory IgA in feces and IgG in serum augmented significantly on day 33 after oral administration. It seemed that surface display of recombinant S. japonicum SjGST on B. subtilis WB600 spores showed good immunogenicity, and B. subtilis spores could be used as potential mucosal delivery vehicles to provide more effective vaccination strategies for parasite prevention and control in the future.
Similar content being viewed by others
Introduction
Schistosomiasis is one of the most prevalent parasitic infections worldwide. An estimated 779 million people are at risk for schistosomiasis, with 207 million infected in 76 countries and territories (Lammie et al. 2006; Steinmann et al. 2006). Approximately 120 million people are symptomatic, and 20 million have severe and debilitating disease (Chitsulo et al. 2000; Engels et al. 2002). Schistosomiasis japonica, caused by the trematode Schistosoma japonicum and characterized by the formation of inflammatory granulomas around the deposited Schistosome eggs, may be more pathogenic compared to other schistosomes affecting humans, due to comparatively higher egg production and deposited organs (Finkelstein et al. 2008). Recent statistics suggested that the infection of schistosomiasis is still growing mainly due to global climate change and ecologic transformations (Zhou et al. 2008c). Furthermore, several types of wild or domestic animals, particularly economically important livestock, such as water buffaloes and pigs, act as reservoir hosts of S. japonicum, and feces of livestock containing S. japonicum eggs are of prime importance for continued transmission of this parasite to humans, which makes the prevention and control of schistosomiasis more difficult. Chemotherapy can be used to control schistosomiasis, but rapid re-infection demands frequent retreatment, which makes this approach expensive. Moreover, the recent observations of strains with decreased drug susceptibility and the transmission of the infection emphasize the need for the development of vaccines for a more long-term approach.
There are already some S. japonicum antigens available currently, e.g., a 26-kDa isoenzyme of S. japonicum glutathione S-transferase (SjGST), which catalyzes detoxification of lipophilic molecules by thioconjugation, is the most effective antigen among the six priority antigens recommended by the World Health Organization for vaccine development (Tiu et al. 1988). According to the immunization studies, recombinant SjGST induced reduction in worm burden and egg production rates of S. japonicum in different animal models (Liu et al. 1995a, b; Xu et al. 1995; Taylor et al. 1998; Wu et al. 2004; Wei et al. 2008); the results seemed quite promising in spite of its not more than 40% protection. Therefore, promotion of protective effect depends on more efficient delivery systems and more efficient vaccinating way.
The development of effective strategy for the mucosal delivery of vaccine antigens has received considerable attention over the past decade, because this way of vaccine administration has a potential to eliminate the requirement for needles and can mimic the natural infection and induce strong immunity against the pathogens (Mann et al. 2009; Ray et al. 2009). However, most protein antigens are poorly immunogenic, when delivered by intra-gastrointestinal route because of the degradation in gastric secretions during gastrointestinal transit, and can induce tolerance (Mann et al. 2009). Novel ways to enhance immune response to antigens have opened up new possibilities for the design of effective mucosal vaccines. Here, we reported one of the effective vaccine delivery systems using Bacillus subtilis spores as a vector.
The Gram-positive bacterium B. subtilis is regarded as a non-pathogen and is classified as a novel food additive which is currently being used as a probiotic for both human and animal consumption (Mazza 1994). The distinguishing feature of B. subtilis is that it produces an endospore as part of its developmental life cycle when starved of nutrients, and the mature spore can survive in a metabolically dormant form indefinitely. The spore offers unique resistance properties which can survive extremes of temperature, desiccation, and exposure to solvents and other noxious chemicals (Mauriello et al. 2004); meanwhile, the spore showed some adjuvant function as well (Barnes et al. 2007), so it would be an attractive vehicle for delivery of heterologous antigens to extreme environments such as the gastrointestinal tract (Duc et al. 2007).
In this work, we introduced probiotic B. subtilis as vehicle based on the use of CotC, a protein component of the spore coat, and Sj26GST protein gene from S. japonicum as candidate to develop an oral vaccine against schistosomiasis. Our work showed that B. subtilis spore surface displaying Sj26GST can elicit both systemic and local immunity against schistosomiasis.
Materials and methods
SjGST protein preparation and antiserum production
The pGEX-4T-1 plasmid carrying sjgst coding sequence was transformed into Escherichia coli strain BL21 (DE3) and was induced by isopropyl-1-thio-β-D-galactopyranoside for 4 h. The bacteria cells were collected by centrifugation and broken by sonication. The SjGST protein was purified by glutathione affinity chromatography (Smith and Joneson 1988) using glutathione Sepharose 4B resin (Pharmacia Biotech) according to the instructions of the manufacturer (Taylor et al. 1998), and the pure protein was stored at −20°C until required.
Purified SjGST protein in Freund’s complete adjuvant was administered by subcutaneous route to per Balb/c 6-week mouse, followed by two boosts at 14-day intervals in Freund’s incomplete adjuvant. Blood was collected at 1 week after the last boost. The antiserums were pooled together stored at −20°C until required. The titer was determined by enzyme-linked-immunosorbent serologic assay (ELISA) for a series of dilution of the serum.
Bacterial strains
WB600 is a protease-deficient B. subtilis strain, which is deficient in six extracellular proteases genes including nprE, aprA, epr, bpf, mpr, and nprB (Wu et al. 1991). All B. subtilis strains used in this study are isogenic derivatives of WB600, which was generally gifted from the genetic engineering laboratory of the Academy of Life Science of Sun Yat-sen University and was employed for proteinic drug production and kept in form of spore.
Construction of recombinant vaccine strain
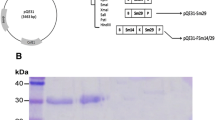
Firstly, we cloned the entire gene of cotC including promoter into plasmid pUS186, which carries a kanamycin resistance gene as constructed before (Zhou et al. 2008a). The forward (cotC-F: 5′-CGGGAATTCTGTAGGATAAATCGTTTGGGC-3′) and reverse (cotC-R: 5′-GGCTCTAGACGGTAGTGTTTTTTATGCTTTTT-3′) primers containing restriction endonuclease sites for EcoRI and XbaI (underlined) were used to amplify the entire cotC gene from B. subtilis WB600 chromosomal DNA. After digestion with EcoRI and XbaI, the amplicon inserted into a similarly restricted pUS186 vector and transformed into B. subtilis WB600 by the competent-cell method (Spizizen 1958).
Next, the S. japonicum 26-KDa sjgst gene encoding sequence (654 bp) was amplified from a plasmid, pGEX-4T-1 (Pharmacia Biotech) that carried the sjgst coding sequence (GeneBank accession no. M14654; AAA57098) using primers (sjgst-F: GGCTCTAGACATGTCCCCTATACTAGG and sjgst-R: GGCAAGCTTTCATTTTGGAGGATGG) containing restriction sites for XbaI and HindIII (underlined). The polymerase chain reaction (PCR) product was digested with XbaI and HindIII and inserted into the pUS186-cotC vector to construct cotC-Sjgst fusion gene, and the ligation product was used for transformation of WB600 competent cells with kanamycin (5 μg/ml) for selection (Spizizen 1958). Fusion gene recombinant plasmid extracted from the positive clone was identified by double restriction enzyme digestion and nucleotide sequencing. One such recombinant strain was identified to express CotC-SjGST fusion protein on spore surface.
Preparation of spores
Sporulation of either CotC or CotC-SjGST spores was made in Difco sporulation medium (DSM) as described (Leighton and Doi 1971; Nicholson and Setlow 1990). Each batch of spores was harvested after 24 h of the initiation of sporulation, treated with lysozyme to break residual sporangial cells, then followed by washing in 1 M NaCl, 1 M KCl, and two times with water. Phenylmethysulfonylfluoride (1 mM) was added to inhibit proteolysis. Spores were treated at 68°C for 1 h to ensure the presence of no viable vegetative cells. The purified spore counts were determined by direct counting with a Bürker chamber under an optical microscope.
Spore coat protein extraction and Western blot analysis
Spore coat proteins were extracted from suspensions of spores at high density (>1 × 1010 spores per milliliter) using a sodium dodecyl sulfate-dithiothreitol (SDS-DTT) extraction buffer as described elsewhere (Nicholson and Setlow 1990). Extracted proteins were subjected to 12% SDS-polyacrylamide gel electrophoresis (PAGE) and visualized by Coomassie brilliant blue G-250 staining used for densitometric analysis by Image J (National Institutes of Health). The proteins were transferred onto a cellulosenitrate membrane after SDS-PAGE. After incubation with mouse anti-SjGST serum, the target protein was identified by horseradish peroxidase-conjugated rabbit anti-mouse antibody (Sigma) and visualized by diaminobenzidine substrate solution.
Immunofluorescence microscopy
Recombinant and native spores were collected and fixed directly using the modified procedure as described by (Harry et al. 1995). Spores, 5 × 106, were fixed with a final concentration of 2.4% paraformaldehyde, 0.04% glutaraldehyde, and 30 mM NaPO4 for 10 min at room temperature. The fixed spores were washed three times in phosphate buffered saline (PBS), followed by re-suspension in GTE solution (50 mM glucose, 20 mM Tris–HCl (pH 7.5), 10 mM ethylenediaminetetraacetic acid, and 2 mg of lysozyme/ml); samples were immediately applied to microscope slide. The dried slides were dipped in −20°C methanol and acetone then waited to dry. Two percent bovine serum albumin in PBS as the blocking solution was added to each slide.
Recombinant spores and native spores were incubated with mouse anti-SjGST sera, respectively, at 1:200 dilution for 45 min at room temperature, washed three times in PBS, and then incubated further with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG; 1:64; Boster) for 45 min at room temperature. After three times washing, samples were used on the microscope. Images were taken using an Olympus IX71 fluorescence microscope equipped with an automatic camera system.
Flow cytometry
A total of 105 purified spores were washed in PBS and incubated for 1 h at 37°C with mouse anti-SjGST sera diluted in 1% bovine serum albumin in PBS. After four washes in PBS, anti-mouse IgG-FITC (1:64; Boster) was added, and the mixture was incubated for 1 h at room temperature (Zhou et al. 2008b). Washed thoroughly, the samples were then resuspended in 500 μl of PBS buffer and analyzed using a FACSCalibur instrument (BD Bioscience, Mountain View, CA, USA) equipped with CellQuest software (BD Bioscience, Mountain View, CA, USA).
Oral immunization and sample collection
Female Balb/c mice (6–8 weeks) were obtained from Animal Center of Sun Yet-sen University (Guangzhou, China) and maintained in our animal care facilities under pathogen-free conditions for the duration of the experiment.
Two groups of eight mice were immunized intra-gastrically (i.g.) with recombinant CotC-SjGST or CotC B. subtilis spores, respectively. One dose consisting of 0.2 ml of the suspension containing 2 × 1010 spores were orally gavages on days 1, 2, 3, 17, 18, 19, 34, 35, and 36. Food and water were removed from mice for 2.5 h before inoculation, respectively. A naive, non-immunized control group was included. Serum and feces samples were collected on days 0, 16, 33, and 51. The fecal pellets were treated following the modified procedure as described elsewhere (Robinson et al. 1997; Zhou et al. 2008a).
ELISA analysis of SjGST-specific IgG, IgG1, IgG2a, and secretary IgA
Briefly, plates were coated with 50 μl of the purified SjGST antigen (5 μg/ml in carbonate-bicarbonate buffer, pH 9.5) per well and blocked with PBS containing 5% heat-inactivated fetal calf serum for 1 h at 37°C. Diluted sera (recombinant spore, native spore immunosera, and naive mice sera, started in 1/40 dilutions) or feces-extracted supernatant 50 μl were added into wells and incubated for 2 h at 37°C. After washing, the plates were incubated with horseradish peroxidase-conjugated second antibodies (1/5,000 dilutions for IgG; 1/2,000 dilutions for IgG1 or IgG2a; 1/2,000 dilutions for IgA) 1 h at 37°C. After washing, the plates were developed with the substrate tetramethylbenzidine 30 min and then stopped by 2 N H2SO4. The absorbance of each well was measured at 450 nm, using PBS sample as a blank control. Each sample was measured in triple wells.
Statistics
Data were expressed as the mean ± SD. Statistical significance was determined by one-way analysis of variance, and Student–Newman–Keuls test was carried out to analyze statistical significance in the experiment groups. A P value of <0.05 was considered significant different. Statistical Package for the Social Sciences (SPSS) for Windows version 13.0 (SPSS, Chicago, IL, USA) was used to analyze the statistics.
Results
Recombinant pUS186-CotC-SjGST expression vector identification
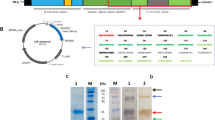
The sjgst in frame with cotC had been proved by sequence analysis and double restriction endonuclease digestion; the Cotc-SjGST fusion protein expression vector was successfully constructed and transformed into the B. subtilis WB600 (Fig. 1).
Identification of the pUS186-cotC and pUS186-cotC-sjgst by polymerase chain reaction (PCR) amplification and double restriction enzyme digestion. a Lane 1: Recombinant pUS186-cotC genes digested by EcoRI and XbaI; Lane 2: PCR production of Bacillus subtilis cotC gene; Lane 3: DNA marker; Arrow points to cotC gene. b Lane 1: Recombinant pUS186-cotC-sjgst genes digested by XbaI and HindIII; Lane 2: PCR production of sjgst gene; Lane 3: DNA marker; Arrow points to sjgst gene
Expression of SjGST in the spore
A densitometric analysis indicated that fusion proteins amounted to 22% of total coat proteins extracted. An average of 0.04 pg of total coat proteins was reproducibly extracted from each spore of the recombinant strain by SDS-DTT treatment as described in detail elsewhere (Mauriello et al. 2004; Zhou et al. 2008a). It was calculated that 8.8 × 10−3 pg of CotC-SjGST fusion protein was extracted from each spore. According to the deduced molecular weight of 34 KDa for the recombinant protein, we estimated that 1.5 × 105 molecules of CotC-SjGST were respectively extracted from each spore. SDS-PAGE analysis of the coat protein fraction extracted from SjGST recombinant spores showed an obvious protein band of approximate 34 KDa in size (Fig. 2a). Western blot with SjGST-specific antiserum also showed a positive band of approximate 34 KDa in spores of SjGST recombinant strain (Fig. 2b).
SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and Western blot analysis of recombinant spore coat proteins extracted from CotC-SjGST strain and CotC strain. a Coomassie blue stained 12% SDS-PAGE of recombinant spore coat protein. Lane 1: protein molecular weight markers; Lane 2: CotC strain; Lane 3: CotC-SjGST expressing strain. Arrow points to fusion protein. b Western blot analysis of proteins extracted from purified spores of strain CotC-SjGST and CotC strain using SjGST-specific mouse antiserum. Lane 1: CotC strain; Lane 2: CotC-SjGST expressing strain. Arrow points to fusion protein
Surface expression of SjGST antigen on the spore surface
By using polyclonal sera against SjGST, we could detect SjGST on recombinant spore surface by specific fluorescent staining after 24 h following the initiation of spore formation (Shown in Fig. 3a). Cytofluorimetric analysis indicated 8,563 positive events out of 10,000 CotC-SjGST recombinant spores analyzed as shown in Fig. 3b.
Detection of presence of SjGST on spore surface by immunofluorescence. Sporulation of Bacillus subtilis strain was induced by Difco sporulation medium. a Fluorescence microscopy image of spores showed CotC-SjGST expressing strain was incubated with mouse anti-SjGST sera, followed by anti-mouse IgG-fluorescein isothiocyanate (FITC), and (b) flow cytometric analysis of SjGST expressing on the spore surface. c Fluorescence microscopy image of spores showed CotC strain was incubated with mouse anti-SjGST sera, followed by anti-mouse IgG-FITC, and (d) flow cytometric analysis the native spores
Serum anti-SjGST responses of mice following oral immunization with recombinant SjGST spores
To test for induction of systemic response, serum samples from groups of eight mice immunized i.g. were examined for the presence of SjGST-specific total IgG. As shown in Fig. 4a, oral immunization of mice with recombinant SjGST spores IgG levers were obtained at days 33 (second dose) and 51 (third dose), significantly above (P < 0.05) those of mice dosed with CotC spores and the naive control group. Our data showed that for oral route, the level of the naive and CotC strain immunizations were not significantly different (P > 0.05).
SjGST-specific serum IgG, IgG1, IgG2a, and fecal sIgA levels (OD450) following intra-gastric immunization with Bacillus subtilis spores. Groups of eight mice were immunized intra-gastrically with recombinant spores expressing CotC-SjGST or CotC spores. Individual samples from each group were tested by enzyme-linked-immunosorbent serologic assay for serum SjGST-specific IgG (a), fecal SjGST-specific IgA (b), and ratio of serum SjGST-specific IgG1/IgG2a (c)
It was further observed that both SjGST-specific IgG1 and IgG2a of recombinant SjGST spores group significantly increased at day 33 and were still increasing at day 51 (data not shown). As shown in Fig. 4c, IgG1 subclass appeared first at day 16 after first dose with 1.19 ± 0.19 times higher than increase difference with IgG2a (P > 0.05). After second dose, the IgG2a subclass increased 1.28 ± 0.16 times (P > 0.05) higher than IgG1 at day 33. At day 51, we detected IgG2a was 1.52 ± 0.23 times (P > 0.05) higher than IgG1. However, we can not detected the significant difference between the two subclasses.
Mucosal anti-SjGST IgA response
Fresh fecal pellets from orally immunized mice were tested for the presence of SjGST-specific secretory IgA (sIgA) by ELISA (Fig. 4b). The levels of fecal SjGST-specific IgA were shown to be significantly higher than those of the control groups (CotC group and naive group, P < 0.05). The sIgA appeared first at day 16 and was still rising till day 33. However, the SjGST-specific sIgA measured in fecal has a significant drop at day 51 (P < 0.05) compared with day 33.
Discussion
Natural infections often induces a strong mucosal and systemic immune response which protect against re-infection, so live attenuated microorganisms are effective vaccine delivery systems because they are replicating organisms that have inherent adjuvant activity (Ryan et al. 2001). More encouraging results have been obtained with recombinant SjGST which induces a pronounced anti-fecundity effect, as well as a moderate but significant level of protection in terms of reduced worm burden (McManus and Bartley 2004; Wu et al. 2005). So, in this paper, we try to increase the antigenicity of recombinant SjGST by alternative adjuvant.
Some attenuated live enteric bacterium, for example, Salmonella typhimurium was used for antigen delivery system (Chabalgoity et al. 2000). It could evoke potent mucosal immunity, but the potential pathogenicity limited its application. Some probiotic bacteria were attractive vaccine vehicle for its safety. We chose the Gram-positive bacterium B. subtilis strain WB600 as vaccine delivery system. It was first applied for expression system of proteinic drugs. To improve the efficiency and stability of heterogenous expression, B. subtilis strain WB600 has been knocked out of six extracellular proteases including neutral protease A, subtilisin, extracellular protease, metalloprotease, bacillopeptidase F, and neutral protease B. WB600 strain only showed 0.32% of the wide-type extracellular protease activity (Wu et al. 1991). A predominant advantage of B. subtilis is the capacity to survive in gastric acid environment in the form of spore as developed in insufficiency of nutrition. The spore itself acts as a good adjuvant for antigen and can protect the protein on its surface from degradation, so it was developed as an antigen surface display vehicle (Isticato et al. 2001).
At least 25 polypeptides have been organized to form the B. subtilis spore coat (Driks 1999). In our experiments, we chose CotC, an 8.8-KDa component of the outer coat, rich in tyrosine (30.3%), lysine (28.8%), and aspartic acid (18.2%) as a fusion partner (Donovan et al. 1987). The strategy to obtain recombinant B. subtilis spores expressing exogenous protein on their surface is the use of B. subtilis cotC gene with its promoter to construct cotC fusions. The PCR amplification product (397 bp) from B. subtilis chromosomal DNA, including cotC gene and its promoter, was cloned into an expression vector pUS186 and constructed a cotC-fusion expression plasmid (Zhou et al. 2008a).
In this study, we expressed S. japonicum 26 kDa GST antigen as a CotC-GST fusion protein in the B. subtilis spores and analyzed the specific systemic and local response of mice mucosally immunized with the recombinant Sj26GST spores.
The immunofluorescence assay demonstrated that recombinant spores displayed SjGST on its surface in CotC-GST fusion protein form. The observed fluorescence was specific (no obvious background was observed with non-recombinant spores). In flow cytometric analysis, the intensity of fluorescence of recombinant spores was significantly greater than the native spore, and the recombination rate was more than 85%. It demonstrated that greater recombination molecule did not affect the structure of spore coat, since most spore structural coat proteins including CotA, CotB, CotC, CotD, and CotF are functionally redundant, and the absence or addition of any one of them does not cause evident phenotypic alterations (Driks 1999). Moreover, the stable structure of the spore coat was potential to stimulate the innate immune system, since cotM (Henriques et al. 1997) and CotP (Reischl et al. 2001) encode proteins with homology to the α-crystalline family of small heat shock proteins, members of which stimulate DC activation (Roelofs et al. 2006).
B. subtilis spores are approximately 1.2 μm in length so are of sufficient size to be taken up by M cells and then transported into the Peyer’s patches where they could interact with macrophages, dendritic cells, or B cells before being transported to the efferent lymph nodes (Duc et al. 2004). Our results showed that CotC-SjGST recombinant spores are immunogenic when following oral administration, because the specific IgA level was significant higher than that of CotC spores. This demonstrated that the SjGST exposed on the surface of spores is in a biologically active form, where its immunogenicity and recombinant spores are able to survive through the stomach barrier without degradation of the SjGST before interaction with the gut-associated lymphoid tissue and transition the mucosa.
In the case of schistosome (blood fluke) worms, two stages of life cycle may be exposed to the host’s mucosa; the larval schistosomulum is exposed to the dermatic and respiratory mucosa, and the egg may come into contact with the intestinal or urinogenital mucosa (Yang et al. 1997). In previous work, it has been determined that mucosal immunization can result in the induction of antibody response at distant mucosal sites, i.e., via a common mucosal immune system (Robinson et al. 2004). Both IgA and some isotypes of IgG have been implicated in protective immunity by neutralizing GST activity (Xu et al. 1991; Grezel et al. 1993) against schistosomiasis in humans and in experimental animal models (Auriault et al. 1990; Grzych et al. 1993). In our results, following i.g. immunization, a rapid increase in SjGST-specific IgA levels could be detected in feces after second dose, although declined by day 51. The significantly level of antigen-specific IgA could detect throughout the study, and the data indicated that the mucosal antibody response fluctuated greatly. This suggested that the mucosal antibody response would not be sustained for very long after vaccination, although immunological memory may ensure a rapid mucosal response to the vaccine antigen upon subsequent re-exposure. As the relative amounts of serum IgG1 and IgG2a antibodies closely matched IL-4 and IFN-gamma cytokine profiles, respectively, it is considered that IgG1 and IgG2a antibody responses are good markers for Th1 and Th2 cytokine expression (Comoy et al. 1998). The present study has confirmed that IgG1 and IgG2a were produced at approximately equal levels following mucosal immunization, which indicates a mixed Th1/Th2 cytokine response in our spore-based model of vaccination using SjGST antigen. Immunizations with recombinant Sm28 GST in aluminum hydroxide only induced a strong Th2-associated antibody (IgG1) and cytokine (IL-4) response (Comoy et al. 1997), and IL-18 as adjuvant induced the Th1-dominant response of Sj26GST DNA vaccine (Wei et al. 2008), while we induced a “balanced” IgG isotype, redolent of a mixed Th1/Th2 profile which was not achieved with aluminum adjuvant or DNA vaccines. The polyfunctionality of induced Th1 and Th2 responses is considered of equal or greater importance for protection. The IgG responses, although not important for protection against schistosomiasis, the induced Th1 response is equally important for protective immune (Hoffmann et al. 1999). Schistosome native antigens only produce partial protection which is mostly due to the bias of stimulating Th2 response (Pearce et al. 2004). Recombinant spores have the potential to alter the immunone response type which renders it promising to be developed as both mucosal and systemic vaccines. The SjGST expressed on the spore surface could induce both systemic and mucosal antibody responses after i.g. administration, suggesting it may be more effective than recombinant SjGST in protection of S. japonicum infection.
Regarded as a non-pathogen and classified as ‘Generally Regarded as Safe’, B. subtilis spores have been administered in the treatment of enteric infections (Mazza 1994), the management of secondary immunodeficiency (Vacca et al. 1983), and used as a probiotic for over 50 years (Novelli et al. 1984; Green et al. 1999). Due to the physical and biological characteristics of the B. subtilis spore, these preparations are extremely resistant and have a prolonged shelf life. In addition, the cost of production of spores for oral bacteriotherapy is much lower than recombinant SjGST purified protein. It is more attractive to be used as oral vaccines for livestock animals in the form of forage additive, particularly the major infection source, bovines, pigs, and ovines.
References
Auriault C, Gras-Masse H, Pierce RJ, Butterworth AE, Wolowczuk I, Capron M, Ouma JH, Balloul JM, Khalife J, Neyrinck JL et al (1990) Antibody response of Schistosoma mansoni-infected human subjects to the recombinant P28 glutathione-S-transferase and to synthetic peptides. J Clin Microbiol 28:1918–1924
Barnes AG, Cerovic V, Hobson PS, Klavinskis LS (2007) Bacillus subtilis spores: a novel microparticle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur J Immunol 37:1538–1547
Chabalgoity JA, Moreno M, Carol H, Dougan G, Hormaeche CE (2000) Salmonella typhimurium as a basis for a live oral Echinococcus granulosus vaccine. Vaccine 19:460–469
Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Trop 77:41–51
Comoy EE, Capron A, Thyphronitis G (1997) In vivo induction of type 1 and 2 immune responses against protein antigens. Int Immunol 9:523–531
Comoy EE, Capron A, Thyphronitis G (1998) Adjuvant is the major parameter influencing the isotype profiles generated during immunization with a protein antigen, the Schistosoma mansoni Sm28-GST. Scand J Immunol 47:444–452
Donovan W, Zhang L, Sandman K, Losick R (1987) Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol 196:1–10
Driks A (1999) Bacillus subtilis spore coat. Microbiol Mol Biol Rev 63:1–20
Duc LH, Hong HA, Uyen NQ, Cutting SM (2004) Intracellular fate and immunogenicity of B. subtilis spores. Vaccine 22:1873–1885
Duc LH, Hong HA, Atkins HS, Flick-Smith HC, Durrani Z, Rijpkema S, Titball RW, Cutting SM (2007) Immunization against anthrax using Bacillus subtilis spores expressing the anthrax protective antigen. Vaccine 25:346–355
Engels D, Chitsulo L, Montresor A, Savioli L (2002) The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop 82:139–146
Finkelstein JL, Schleinitz MD, Carabin H, McGarvey ST (2008) Decision-model estimation of the age-specific disability weight for schistosomiasis japonica: a systematic review of the literature. PLoS Negl Trop Dis 2:e158
Green DH, Wakeley PR, Page A, Barnes A, Baccigalupi L, Ricca E, Cutting SM (1999) Characterization of two Bacillus probiotics. Appl Environ Microbiol 65:4288–4291
Grezel D, Capron M, Grzych JM, Fontaine J, Lecocq JP, Capron A (1993) Protective immunity induced in rat schistosomiasis by a single dose of the Sm28GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol 23:454–460
Grzych JM, Grezel D, Xu CB, Neyrinck JL, Capron M, Ouma JH, Butterworth AE, Capron A (1993) IgA antibodies to a protective antigen in human Schistosomiasis mansoni. J Immunol 150:527–535
Harry EJ, Pogliano K, Losick R (1995) Use of immunofluorescence to visualize cell-specific gene-expression during sporulation in Bacillus subtilis. J Bacteriol 177:3386–3393
Henriques AO, Beall BW, Moran CP Jr (1997) CotM of Bacillus subtilis, a member of the alpha-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol 179:1887–1897
Hoffmann KF, James SL, Cheever AW, Wynn TA (1999) Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol 163:927–938
Isticato R, Cangiano G, Tran HT, Ciabattini A, Medaglini D, Oggioni MR, De Felice M, Pozzi G, Ricca E (2001) Surface display of recombinant proteins on Bacillus subtilis spores. J Bacteriol 183:6294–6301
Lammie PJ, Fenwick A, Utzinger J (2006) A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol 22:313–321
Leighton TJ, Doi RH (1971) The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem 246:3189–3195
Liu S, Song G, Xu Y, Yang W, McManus DP (1995a) Immunization of mice with recombinant Sjc26GST induces a pronounced anti-fecundity effect after experimental infection with Chinese Schistosoma japonicum. Vaccine 13:603–607
Liu S, Song GC, Xu YX, Yang W, McManus DP (1995b) Anti-fecundity immunity induced in pigs vaccinated with recombinant Schistosoma japonicum 26 kDa glutathione-S-transferase. Parasite Immunol 17:335–340
Mann JF, Acevedo R, Campo JD, Perez O, Ferro VA (2009) Delivery systems: a vaccine strategy for overcoming mucosal tolerance? Expert Rev Vaccines 8:103–112
Mauriello EM, Duc LH, Isticato R, Cangiano G, Hong HA, De Felice M, Ricca E, Cutting SM (2004) Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 22:1177–1187
Mazza P (1994) The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm 133:3–18
McManus DP, Bartley PB (2004) A vaccine against Asian schistosomiasis. Parasitol Int 53:163–173
Nicholson W, Setlow P (1990) Sporulation, germination and outgrowth. In: Harwood CR, Cutting SM (eds) Molecular biological methods for Bacillus. Wiley, Chichester, pp 391–450
Novelli A, Ulivelli A, Reali EF, Mannelli F, Trombi Belcari L, Spezia R, Periti P (1984) Bacillus subtilis spores as a natural pro-host oral agent. Preliminary data in children. Chemioterapia 3:152–155
Pearce EJ, MK C, Sun J, JT J, McKee AS, Cervi L (2004) Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev 201:117–126
Ray HJ, Cong Y, Murthy AK, Selby DM, Klose KE, Barker JR, Guentzel MN, Arulanandam BP (2009) Oral live vaccine strain induced protective immunity against pulmonary Francisella tularensis challenge is mediated by CD4+ T cells and antibodies including IgA. Clin Vaccine Immunol 16:444–452
Reischl S, Thake S, Homuth G, Schumann W (2001) Transcriptional analysis of three Bacillus subtilis genes coding for proteins with the alpha-crystallin domain characteristic of small heat shock proteins. FEMS Microbiol Lett 194:99–103
Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW (1997) Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol 15:653–657
Robinson K, Chamberlain LM, Lopez MC, Rush CM, Marcotte H, Le Page RW, Wells JM (2004) Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect Immun 72:2753–2761
Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176:7021–7027
Ryan EJ, Daly LM, Mills KH (2001) Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol 19:293–304
Smith DB, Joneson KS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31–41
Spizizen J (1958) Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleotide. Proc Natl Acad Sci U S A 44:1027–1078
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6:411–425
Taylor M, Huggins MC, Shi F, Lin J, Tian E, Ye P, Shen W, Qian CG, Lin BF, Bickle QD (1998) Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine 16:1290–1298
Tiu WU, Davern KM, Wright MD, Board PG, Mitchell GF (1988) Molecular and serological characteristics of the glutathione S-transferases of Schistosoma japonicum and Schistosoma mansoni. Parasite Immunol 10:693–706
Vacca A, Pantaleo G, Ronco M, Dammacco F (1983) Chemotherapy for multiple myeloma using an intermitenet combination drug schedule (melphalon + prednisone) and alternating course of B. subtilis spores. Chemioterapia 2:300–305
Wei F, Liu Q, Gao S, Shang L, Zhai Y, Men J, Jiang L, Zhu XQ, Fu Z, Shi Y, Xia Z, Lin J (2008) Enhancement by IL-18 of the protective effect of a Schistosoma japonicum 26 kDa GST plasmid DNA vaccine in mice. Vaccine 26:4145–4149
Wu XC, Lee W, Tran L, Wong SL (1991) Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J Bacteriol 173:4952–4958
Wu Z, Liu S, Zhang S, Tong H, Gao Z, Liu Y, Lin D, Liu Z, Wu G, Yi H, Song G, Xu Y (2004) Persistence of the protective immunity to Schistosoma japonicum in Chinese yellow cattle induced by recombinant 26 kDa glutathione-S-transferase (reSjc26GST). Vet Parasitol 123:167–177
Wu ZD, Lu ZY, Yu XB (2005) Development of a vaccine against Schistosoma japonicum in China: a review. Acta Trop 96:106–116
Xu CB, Verwaerde C, Grzych JM, Fontaine J, Capron A (1991) A monoclonal antibody blocking the Schistosoma mansoni 28-kDa glutathione S-transferase activity reduces female worm fecundity and egg viability. Eur J Immunol 21:1801–1807
Xu S, Shi F, Shen W, Lin J, Wang Y, Ye P, Tian E, Qian C, Lin B, Shi Y et al (1995) Vaccination of sheep against Schistosoma japonicum with either glutathione S-transferase, keyhole limpet haemocyanin or the freeze/thaw schistosomula/BCG vaccine. Vet Parasitol 58:301–312
Yang W, Gobert GN, McManus DP (1997) Oral vaccination of mice with recombinant Schistosoma japonicum proteins induces specific anti-parasite antibodies and damage to adult worms after a challenge infection. Int J Parasitol 27:843–853
Zhou Z, Xia H, Hu X, Huang Y, Li Y, Li L, Ma C, Chen X, Hu F, Xu J, Lu F, Wu Z, Yu X (2008a) Oral administration of a Bacillus subtilis spore-based vaccine expressing Clonorchis sinensis tegumental protein 22.3 kDa confers protection against Clonorchis sinensis. Vaccine 26:1817–1825
Zhou Z, Xia H, Hu X, Huang Y, Ma C, Chen X, Hu F, Xu J, Lu F, Wu Z, Yu X (2008b) Immunogenicity of recombinant Bacillus subtilis spores expressing Clonorchis sinensis tegumental protein. Parasitol Res 102:293–297
Zhou X, Yang GJ, Yang K, Wang XH, Hong QB, Sun LP, Malone JB, Kristensen TK, Bergquist NR, Utzinger J (2008c) Potential impact of climate change on schistosomiasis transmission in China. Am J Trop Med Hyg 78:188–194
Acknowledgments
We thank Dr. Bin Li for the help in revising the manuscript. This work was supported by grants from National Natural Science Foundation of China (no. 30671831) and the National High Technology Research and Development Program of China (863 program; no. 2006AA02Z422).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the national Natural Science Foundation of China (No.30671831) and the National High Technology Research and Development Program of China (863 program; No.2006AA02Z422).
Rights and permissions
About this article
Cite this article
Li, L., Hu, X., Wu, Z. et al. Immunogenicity of self-adjuvanticity oral vaccine candidate based on use of Bacillus subtilis spore displaying Schistosoma japonicum 26 KDa GST protein. Parasitol Res 105, 1643–1651 (2009). https://doi.org/10.1007/s00436-009-1606-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1606-7