Abstract
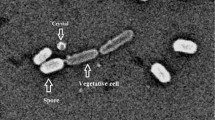
The tremendous worldwide efforts to isolate novel mosquito larvicidal bacteria with improved efficacy present significant promise to control vector-borne diseases of public health importance. In the present study, two native bacterial isolates, Bacillus thuringiensis (Bt SV2) and Serratia species (SV6) were evaluated for mosquito larvicidal potential against the early fourth instar larvae of Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus with reference to B. thuringiensis subsp. israelensis (Bti) H 14. The native Gram-positive, spore-forming Bt SV2 isolate showed 100% mortality against early fourth instars of Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus, in parallel to Bti H14 strain. After 24 h, Bt SV2 showed 98%, 89%, and 80.67%, and Bti H14 showed 92%, 98.33%, and 60% mortality against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus, respectively. Serratia SV6 showed highest activity against Culex quinquefasciatus (100%) followed by Anopheles stephensi (95%) and Aedes aegypti (91%) after 48 h of exposure. The Gram-negative Serratia SV6 showed delayed toxicity compared to Bti H14 and Bt SV2 against early fourth instars of Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus. The relative mortality of all treatments after 12-h exposures showed the varied toxicity with respect to exposure time, bacterial treatment, and mosquito species. Genetic relatedness of the strains was confirmed on the basis of phylogenetic reconstructions based on alignment of 16S rRNA gene sequences which indicated a strong clustering of the strain SV2 with B. thuringiensis and the strain SV6 with Serratia nematodiphila. In conclusion, the native isolate B. thuringiensis SV2 showed significant toxicity while Serratia SV6 showed less and delayed toxicity against several mosquito species compared with BtiH14. They may be used as novel bacterial insecticidal agents in mosquito vector-borne disease control. To our knowledge, this is the first report on mosquito larvicidal potential of Serratia species.




Similar content being viewed by others
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–266
Amer A, Mehlhorn H (2006a) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006b) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Aucken HM, Pitt TL (1998) Antibiotic resistance and putative virulence factors of Serratia marcescens with respect to O and K serotypes. J Med Microbiol 47:1105–1113
Balaraman K (1995) Mosquito control potential of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus. ICMR Bull 25:45–51
Bernhard K, Jarrett P, Meadows M, Butt J, Ellis DJ, Roberts GM, Pauli S, Rodgers P, Burges HD (1997) Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization, and activity against insect pests. J Invertebr Pathol 70:59–68
Brownbridge M, Onyango T (1992) Screening of exotic and locally isolated Bacillus thuringiensis (Berliner) strains in Kenya for toxicity of the spotted stem borer Chilo partellus (swinhoe). Trop Pest Manag 38:71–81
Carozzi NB, Kramer VC, Warren GW, Evola S, Koziel MG (1991) Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl Environ Microbiol 57:3057–3061
Chaufaux J, Marchal M, Gilois N, Jehanno I, Buisson C (1997) Investigation of natural strains of Bacillus thuringiensis in different biotypes throughout the world. Can J Microbiol 43:337–343
Chenniappan K, Ayyadurai N (2011) Synergistic activity of Cyt1A from Bacillus thuringiensis subsp. israelensis with Bacillus sphaericus B101H5a5b against Bacillus sphaericus B101 H5a5b-resistant strains of Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res. doi:10.1007/s00436-011-2502-5
Damgaard PH, Abdel-Hameed A, Eilenberg J, Smits P (1998) Natural occurrence of Bacillus thuringiensis on grass foliage. World J Microbiol Biotechnol 14:239–242
de Barjac H (1978) Une nouvelle varie‘te’ de Bacillus thuringiensis tre’s toxique pour les moustiques: B. thuringiensis var. israelensis se’rotype 14. C R Acad Sci Se’r D 286:797–800
Feitelson JS, Payne J, Kim L (1992) Bacillus thuringiensis: insects and beyond. Nat Biotechnol 10:271–275
Ferré J, Van Rie J (2002) Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 47:501–533
Forcada C, Alcacer E, Garcera MD, Tato A, Martinez R (1999) Resistance to Bacillus thuringiensis Cry1Ac toxin in three strains of Heliothis virescens: proteolytic and SEM study of the larval midgut. Arch Insect Biochem Physiol 42:51–63
Forsyth G, Logan NA (2000) Isolation of Bacillus thuringiensis from Northern Victoria Land, Antarctica. Lett Appl Microbiol 30:263–266
Gahan LJ, Gould F, Heckel DG (2001) Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293:857–860
Goldberg LY, Margalit J (1977) A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univittatus, Aedes aegypti and Culex pipiens. Mosq News 37:355–358
Grimont PAD, Grimont F (1978) Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol 8:73–83
Grimont PAD, Grimont F, Dulongderosnay HLC, Sneath PHA (1977) Taxonomy of the genus Serratia. J Gen Microbio 98:39–66
Hines DA, Saurugger PN, Ihler GM, Benedik MJ (1988) Genetic analysis of extracellular proteins of Serratia marcescens. J Bacteriol 170:4141–4146
Hossain MA, Ahmed S, Hoque S (1997) Abundance and distribution of Bacillus thuringiensis in the agricultural soil of Bangladesh. J Invertebr Pathol 70:221–225
Jackson TA, Boucias DG, Thaler JO (2001) Pathobiology of amber disease, caused by Serratia spp., in the New Zealand grass grub, Costelytra zealandica. J Invertebr Pathol 4:232–243
Jeong HU, Mun HY, Oh HK, Kim SB, Yang KY, Kim I, Lee HB (2010) Evaluation of Insecticidal Activity of a Bacterial Strain, Serratia sp. EML-SE1 against Diamondback Moth. J Microbiol 48(4):541–545
Kaufman PE, Mann RS, Butler JF (2011) Insecticidal potency of novel compounds on multiple insect species of medical and veterinary importance. Pest Manag Sci 67:26–35
Kim Y, Kim K, Seo J, Shrestha S, Kim HH, Nalini M, Yi Y (2009) Identification of an entomopathogenic bacterium, Serratia sp. ANU101, and its hemolytic activity. J Microbiol Biotechnol 19(3):314–322
Kovendan K, Murugan K, Vincent S, Barnard DR (2011a) Studies on larvicidal and pupicidal activity of Leucas aspera Willd. (Lamiaceae) and bacterial insecticide, Bacillus sphaericus, against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res. doi:10.1007/s00436-011-2469-2
Kovendan K, Murugan K, Vincent S, Kamalakannan S (2011b) Larvicidal efficacy of Jatropha curcas and bacterial insecticide, Bacillus thuringiensis, against lymphatic filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res. doi:10.1007/s00436-011-2368-6
Kurz CL, Chauvet S, Andres E, Aurouze M, Vallet I, Michel GP et al (2003) Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J 22:1451–1460
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lecadet MM, Frachon E, Cosmao Dumanoir V, Ripouteau H, Hamon S, Laurent P, Thiéry I (1999) Updating the H-antigen classification of Bacillus thuringiensis. J Appl Microbiol 86:660–762
Lo’pez-Meza J, Federici BA, Poehner WJ, Martinez-Castillo A, Ibarra JE (1995) Highly mosquitocidal isolates of Bacillus thuringiensis subspecies kenyae and entomocidus from Mexico. Biochem Syst Ecol 23:461–468
Lysyk TJ, Kalischuk-Tymensen LD, Selinger LB (2002) Comparsion of selected growth media for culturing Serratiamarcescens, Aeromonas sp., and Pseudomonas aeruginosa as pathogens of adult Stomoxys calcitrans (Diptera: Muscidae). J Med Entomol 39:89–98
Martin PAW, Travers RS (1989) worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol 55:2437–2442
Mwangangi JM, Kahindi SC, Kibe LW, Nzovu JG, Luethy P, Githure JI, Mbogo CM (2011) Wide-scale application of Bti/Bs biolarvicide in different aquatic habitat types in urban and peri-urban Malindi, Kenya. Parasitol Res 108:1355–1363
Nunez-Valdez ME, Calderon MA, Aranda E, Hernandez L, Ramirez-Gama RM, Lina L, Rodriguez-Segura Z, Gutierrez MC, Villalobos FJ (2008) Identification of a putative Mexican strain of Serratia entomophila pathogenic against root-damaging larvae of Scarabaeidae (Coleoptera). Appl Environ Microbiol 74:802–810
Oppert B, Kramer KJ, Beeman RW, Johnson D, McGaughey WH (1997) Proteinase-mediated insect resistance to Bacillus thuringiensis toxins. J Biol Chem 272:23473–23476
Orduz S, RojasW CMM, Montoya AE, de Barjac H (1992) A new serotype of Bacillus thuringiensis from Colombia toxic to mosquito larvae. J Invertebr Pathol 59:99–103
Padua LE, Ohba M, Aizawa K (1984) Isolation of a Bacillus thuringiensis strain (serotype 8a:8b) highly and selectively toxic against mosquito larvae. J Invertebr Pathol 44:12–17
Park H-W, Hayes SR, Mangum CM (2008) Distribution of mosquitocidal Bacillus thuringiensis and Bacillus sphaericus from sediment samples in Florida. J Asia-Pac Entomol 11:217–220
Patil SV, Patil CD, Salunke BK, Salunkhe RB (2010) Larvicidal efficacy of six plants against two mosquito species Aedes aegypti and Anopheles stephensi. Trop Biomed 27(3):360–365
Patil CD, Patil SV, Salunke BK, Salunkhe RB (2011a) Bioefficacy of Plumbago zeylanica (Plumbaginaceae) and Cestrum nocturnum (Solanaceae) plant extracts against Aedes aegypti (Diptera: Culicide) and nontarget fish Poecilia reticulata. Parasitol Res 108(5):1253–1263
Patil CD, Patil SV, Salunke BK, Salunkhe RB (2011b) Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol Res. doi:10.1007/s00436-011-2365-9
Ragni A, Thie’ry I, Dele’cluse A (1996) Characterization of six highly mosquitocidal Bacillus thuringiensis strains that do not belong to H-14 serotype. Curr Microbiol 32:48–54
Redwane A, Lazrek HB, Bouallam S, Markouk M, Amarouch H, Jana M (2002) Larvicidal activity of extracts from Querus Lusitania var infectoria galls (Oliv). J Ethnopharmacol 79:261–263
Salunkhe RB, Patil SV, Patil CD, Salunke BK (2011) Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol Res. doi:10.1007/s00436-011-2328-1
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806
Seleena P, Lee HL, Lecadet M-M (1995) A new serovar of Bacillus thuringiensis possessing 28a28c flagellar antigenic structure: Bacillus thuringiensis serovar jegathesan, selectively toxic against mosquito larvae. J Am Mosq Control Assoc 11:471–473
Sikorowski PP, Lawrence AM, Inglis GD (2001) Effects of Serratia marcescens on rearing of the tobacco budworm (Lepidoptera: Noctuidae). Am Entomol 47:51–60
Singh G, Prakash S (2009) Efficacy of Bacillus sphaericus against larvae of malaria and filarial vectors: an analysis of early resistance detection. Parasitol Res 104:763–766
Smith RA, Couche GA (1991) The phylloplane as a source of Bacillus thuringiensis variants. Appl Environ Microbiol 57:311–315
Steinhaus EA (1959) Serratia marcescens Bizio as an insect pathogen. Hilgardia 28:351–380
Su T, Mulla MS (1998) Ovicidal activity of neem products (Azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J Am Mosq Control Assoc 14:204–209
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Walther CJ, Couche GA, Pfannenstiel MA, Egan SE, Bivin LA, Nickerson KW (1986) Analysis of mosquito larvicidal potential exhibited by vegetative cells of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol 52(4):650–653
Xia X, Xie Z (2001) DAMBE: software package for data analysis in molecular biology and evolution. J Hered 92:371–373
Acknowledgments
Authors would like to express their deep gratitude to Dr. Yogesh S. Shouche, Scientist F, National Centre for Cell Sciences (NCCS), Pune, India for 16S rRNA identification of bacterial samples.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, C.D., Patil, S.V., Salunke, B.K. et al. Insecticidal potency of bacterial species Bacillus thuringiensis SV2 and Serratia nematodiphila SV6 against larvae of mosquito species Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus . Parasitol Res 110, 1841–1847 (2012). https://doi.org/10.1007/s00436-011-2708-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2708-6