Abstract
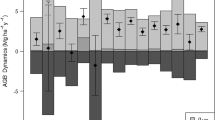
There are a number of controversies surrounding both biomass estimation and carbon balance in tropical forests. Here we use long-term (from 1978 through 2000) data from five 0.5-ha permanent sample plots (PSPs) within a large tract of relatively undisturbed Atlantic moist forest in southeastern Brazil to quantify the biomass increment (ΔMI), and change in total stand biomass (ΔMstand), from mortality, recruitment, and growth data for trees ≥10 cm diameter at breast height (DBH). Despite receiving an average of only 1,200 mm annual precipitation, total forests biomass (334.5±11.3 Mg ha−1) was comparable to moist tropical forests with much greater precipitation. Over this relatively long-term study, forest biomass experienced rapid declines associated with El Niño events, followed by gradual biomass accumulation. Over short time intervals that overlook extreme events, these dynamics can be misinterpreted as net biomass accumulation. However for the 22 years of this study, there was a small reduction in forest biomass, averaging −1.2 Mg ha−1 year−1 (±3.1). Strong climatic disturbances can severely reduce forest biomass, and if the frequency and intensity of these events increases beyond historical averages, these changing disturbance regimes have the capacity to significantly reduce forest biomass, resulting in a net source of carbon to the atmosphere.





Similar content being viewed by others
References
Aiba SI, Kitayama K (2002) Effects of the 1997–98 El Nino drought on rain forests of Mount Kinabalu, Borneo. J Trop Ecol 18:215–230
Baker TR, Burslem DFRP, Swaine MD (2003) Variation in tropical forest growth rates: combined effects of functional group composition and resource availability. Perspect Plant Ecol Evol Syst 6:21–36
Baker TR, Phillips OL, Malhi Y, Almeida S, Arroyo L, Di Fiore A, Killeen T, Laurance SG, Laurance WF, Lewis SL, Lloyd J, Monteagudo A, Neill DA, Patiño S, Pitman NCA, Silva N, Vásquez Martínez R (2004) Increasing biomass in Amazonian forest plots? Phil Trans R Soc London B 359:353–365
Brown IF, Martinelli LA, Thomas WW, Moreira MZ, Ferreira CAC, Victoria RA (1995) Uncertainty in the biomass of Amazonian forests: an example from Rondônia, Brazil. For Ecol Manage 75:175–189
Chambers JQ, Silver WL (2004) Some aspects of ecophysiological and biogeochemical responses of tropical forests to atmospheric change. Phil Trans R Soc London B 359:463–476
Chambers JQ, dos Santos J, Ribeiro RJ, Higuchi N (2001) Tree damage, allometric relationships, and above-ground net primary production in central Amazon forest. For Ecol Manage 152:73–84
Chambers JQ, Tribuzy ES, Toledo LC, Crispim BF, Higuchi N, dos Santos J, Araújo AC, Kruijt B, Nobre AD, Trumbore SE (2004a) Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecol Appl 14:72–88
Chambers JQ, Higuchi N, Teixeira LM, dos Santos J, Laurance SG, Trumbore SE (2004b) Response of tree biomass and wood litter to disturbance in a Central Amazon forest. Oecologia (in press)
Chave J, Riera B, Dubois MA (2001) Estimation of biomass in a neotropical forest of French Guiana: spatial and temporal variability. J Trop Ecol 17:79–96
Chave J, Condit R, Lao S, Caspersen JP, Foster RB, Hubbell SP (2003) Spatial and temporal variation in biomass of a tropical forest: results from a large census plot in Panama. J Ecol 91:240–252
Clark DA (2002) Are tropical forests an important carbon sink? Reanalysis of the long-term plot data. Ecol Appl 12:3–7
Clark DA (2004) Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Phil Trans R Soc London B 359:477–491
Clark DB, Clark DA (1996) Abundance, growth and mortality of very large trees in neotropical lowland rain forest. For Ecol Manage 80:235–244
Clark DB, Clark DA, Rich PM (1993) Comparative analysis of microhabitat utilization by saplings of nine tree species in neotropical rain forest. Biotropica 25:397–407
Clark DB, Clark DA, Read JM (1998) Edaphic variation and the mesoscale distribution of tree species in a neotropical rain forest. J Ecol 86:101–112
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J, Holland EA. (2001a) Net primary production in tropical forests: an evaluation and synthesis of existing field data. Ecol Appl 11:371–384
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J (2001b) Measuring net primary production in forests: concepts and field methods. Ecol Appl 11:356–370
Clark DA, Piper SC, Keeling CD, Clark DB (2003) Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc Natl Acad Sci USA 100:5852–5857
Clinebell RR II, Phillips OL, Gentry AH, Stark N, Zuuring H (1995) Prediction of neotropical tree and liana species richness from soil and climatic data. Biodivers Conserv 4:56–90
Cochran WG (1977) Sampling techniques. Wiley, New York
Condit R, Hubbell SP, Foster RB (1992) Short-term dynamics of a neotropical forest. Bioscience 42:822–828
Condit R, Hubbell SP, Foster RB (1995) Mortality rates of 205 neotropical trees and shrub species and the impact of a severe drought. Ecol Monogr 65:419–439
Condit R, Hubbell SP, Foster RB (1996) Changes in tree species abundance in a neotropical forest over eight years: impact of climate change. J Trop Ecol 12:231–256
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wsniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Engel VL (2000) Estudo Fenológico de Espécies Arbóreas de uma Floresta Tropical em Linhares—ES. PhD Thesis, Campinas University
Fearnside PM (2000) Global warming and tropical land-use change: greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation. Clim Change 46:115–158
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Gale N, Barford AS (1999) Canopy tree mode of death in a western Ecuadorian rain forest. J Trop Ecol 15:415–436
Garay I, Kindel A, Jesus RM (1995) Diversity of humus forms in the Atlantic forest ecosystems (Brazil): the tableland Atlantic forest. Acta Oecol 16:553–570
Grace J, Lloyd J, McIntyre J, Miranda AC, Meir P, Miranda HS, Nobre C, Moncrieff JM, Massheder J, Malhi Y, Wright IR, Gash J (1995). Carbon dioxide uptake by an undisturbed tropical rain forest in Southwest Amazonia, 1992–93. Science 270:778–780
Hartshorn GS (1990) An overview of neotropical forest dynamics. In Gentry AH (ed) Four neotropical rainforests. Yale University Press, New Haven, pp 585–599
IPCC (2001) Climate change 2001: the scientific basis. Contribution of working group I to the third assessment report of the international panel on climate change. Cambridge University Press, Cambridge
Jesus RM (2001) Manejo florestal: impactos da exploração na estrutura da floresta e sua sustentabilidade econômica. PhD Thesis, Campinas University
Jesus RM, Rolim SG (2004) Fitossociologia da floresta atlântica de tabuleiro. Boletim SIF (in press)
Laurance WF, Laurance SG, Ferreira LV, Rankin-de-Merona JM, Gascon C, Lovejoy TE (1997) Biomass collapse in Amazonian forest fragments. Science 278:1117–1118
Laurance WF, Fearnside PM, Laurance SG, Delamonica P, Lovejoy TE, Rankin-de Merona JM, Chambers JQ, Gascon C (1999) Relationship between soils and Amazon forest biomass: a landscape-scale study. For Ecol Manage 118:127–138
Laurance WF, Williamson GB, Delamonica P, Oliveira AA, Lovejoy TE, Gascon C, Pohl L (2001) Effects of a strong drought on Amazonian forest fragments and edges. J Trop Ecol 17:771–785
Leigh EG Jr, Windsor DM, Rand AS, Foster RB (1990) The impact of the “El Niño” drought of 1982–83 on a Panamanian semideciduous forest. In: Glynn PW (ed) Global ecological consequences of the 1982–83 El Nino-Southern Oscillation. Elsevier, Amsterdam, pp 473–486
Leighton M, Wirawan N (1986) Catastrophic drought and fire in Borneo tropical rain forest associated with the 1982–83 El Niño Southern Oscillation event. In: Prance GT (ed) Tropical forests and the world atmosphere. West View, Boulder, pp 75–102
Lewis SL, Phillips OL, Baker TR, Lloyd J, Malhi Y, Almeida S, Higuchi N, Laurance WF, Neill DA, Silva JNM, Terborgh J, Torres Lezama A, Vásquez Martínez R, Brown S, Chave J, Kuebler C, Nũńez Vargas P, Vinceti B (2004) Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Phil Trans R Soc London B 359:421–436
Lugo AE, Scatena FN (1996) Background and catastrophic tree mortality in tropical moist, wet, and rain forests. Biotropica 28:585–599
Malhi Y, Grace J (2000) Tropical forests and atmospheric carbon dioxide. Trends Ecol Evol 15:332–337
Malhi Y, Nobre AD, Grace J, Kruijt B, Pereira MGP, Culf A, Scott S (1998) Carbon dioxide transfer over a Central Amazonian rain forest. J Geophys Res D 103:31593–31612
Malhi Y, Meir P, Brown S (2002) Forests, carbon and global climate. Phil Trans R Soc London A 360:1567–1591
Melillo JM, McGuire AD, Kicklighter DW, Moore B III, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Nakagawa M, Tanaka K, Nakashizuka T, Ohkubo T, Kato T, Maeda T, Sato K, Miguchi H, Nagamasu H, Ogino K, Teo S, Hamid AA, Seng LH (2000) Impact of severe drought associated with the 1997–98 El Niño in a tropical forest in Sarawak. J Trop Ecol 16:355–367
Nascimento HEM, Laurance WF (2002) Total above-ground biomass in central Amazonian rainforests: a landscape-scale study. For Ecol Manage 168:311–321
Nascimento HEM, Laurance WF (2004) Biomass dynamics in Amazonian forest fragments. Ecol Appl 14:127–138
Peixoto AL, Gentry A (1990) Diversidade e composição florística da mata de tabuleiro na Reserva Florestal de Linhares (Espírito Santo, Brasil). Rev Bras Bot 13:19–25
Peixoto AL, Silva IM (1997) Tabuleiro forests of northern Espirito Santo, South-eastern Brazil. In: Davis SD, Heywood VH, Herrera-McBryde O, Villa-Lobos J, Hamilton AC (eds) Centres of plant diversity: a guide and strategy for their conservation, vol 3. The Americas, WWF/IUCN, Cambridge, pp 369–372
Peixoto AL, Silva IM, Pereira OJ, Simonelli M, Rolim SG (2004) Tabuleiro forests north of the rio Doce: their representation in the Vale do Rio Doce Natural Reserve, Espírito Santo, Brazil. Mem New York Bot Gard (in press)
Phillips OL (1996) Long-term environmental change in tropical forests: increasing tree turnover. Environ Conserv 23:235–248
Phillips OL, Gentry AH (1994) Increasing turnover through time in tropical forests. Science 263:954–958
Phillips OL, Malhi Y, Higuchi N, Laurance WF, Nunez PV, Vasquez RM, Laurance SG, Ferreira LV, Stern M, Brown S, Grace J (1998) Changes in the carbon balance of tropical forests: evidence from long-term plots. Science 282:439–442
Phillips OL, Malhi Y, Vinceti B, Baker T, Lewis SL, Higuchi N, Laurance WF, Vargas PN, Martinez RV, Laurance S, Ferreira LV, Stern M, Brown S, Grace J (2002a) Changes in growth of tropical forests: evaluating potential biases. Ecol Appl 12:576–587
Rolim SG, Chiarello AG (2004) Slow death of Atlantic forest trees in cocoa agroforestry in southeastern Brazil. Biodiversity Conserv (in press)
Rolim SG, Couto HTZ, Jesus RM (1999) Mortalidade e recrutamento de árvores na floresta atlântica em Linhares (ES). Sci For 55:49–69
Rolim SG, Couto HTZ, Jesus RM (2001) Fluctuaciones temporales en la composición florística del bosque tropical atlántico. Biotropica 33:12–22
Saldarriaga JG, West DC, Tharp ML, Uhl C (1988) Long-term chronosequence in the upper rio Negro of Colombia and Venezuela. J Ecol 76:938–958
Saleska SR, Miller SD, Matross DM, Goulden ML, Wofsy SC, da Rocha HR, de Camargo PB, Crill P, Daube BC, de Freitas HC, Hutyra L, Keller M, Kirchhoff V, Menton M, Munger JW, Pyle EH, Rice AH, Silva H (2003) Carbon in Amazon forests: unexpected seasonal fluxes and disturbance-induced losses. Science 302:1554–1557
Sheil D, May R (1996) Mortality and recruitment rate evaluations in heterogeneous tropical forests. J Ecol 84:91–100
Swaine MD (1989) Population dynamics of tree species in tropical forests. In: Holm-Nielsen LB, Nielsen IC, Balslev H (Eds) Tropical forests: botanical, dynamics, speciation and diversity. Academic, London, pp 101–109
Williamson GB, Laurance WF, Oliveira AA, Delamonica P, Gascon C, Lovejoy TE, Pohl L (2000) Amazonian tree mortality during the 1997 El Nino drought. Conserv Biol 14:1538–1542
Acknowledgements
We are grateful to the Companhia Vale do Rio Doce for providing research support. We also thank C. Zartman and two anonymous reviewers for many constructive comments and suggestions on the manuscript, as well as all field technicians for their efforts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rolim, S.G., Jesus, R.M., Nascimento, H.E.M. et al. Biomass change in an Atlantic tropical moist forest: the ENSO effect in permanent sample plots over a 22-year period. Oecologia 142, 238–246 (2005). https://doi.org/10.1007/s00442-004-1717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1717-x