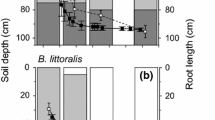
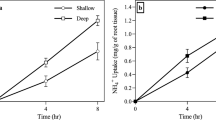
Abstract
While seasonal redistribution of fine root biomass in response to fluctuations in groundwater level is often inferred in phreatophytic plants, few studies have observed the in situ growth dynamics of deep roots relative to those near the surface. We investigated the root growth dynamics of two Banksia species accessing a seasonally dynamic water table and hypothesized that root growth phenology varied with depth, i.e. root growth closest to the water table would be influenced by water table dynamics rather than surface micro-climate. Root in-growth bags were used to observe the dynamics of root growth at different soil depths and above-ground growth was also assessed to identify whole-plant growth phenology. Root growth at shallow depths was found to be in synchrony with above-ground growth phenophases, following increases in ambient temperature and soil water content. In contrast, root growth at depth was either constant or suppressed by saturation. Root growth above the water table and within the capillary fringe occurred in all seasons, corresponding with consistent water availability and aerobic conditions. However, at the water table, a seasonal cycle of root elongation with drawdown in summer followed by trimming in response to water table rise and saturation in winter, was observed. The ability to grow roots year-round at the capillary fringe and redistribute fine root biomass in response to groundwater drawdown is considered critical in allowing phreatophytes, in seasonally water-limited environments, to maintain access to groundwater throughout the year.




Similar content being viewed by others
References
Bell D, Stephens L (1984) Seasonality and phenology of Kwongan species. In: Pate J, Beard J (eds) Kwongan plant life of the sand plain. University of Western Australia Press, Perth, pp 205–226
Böhm W (1979) Methods of studying root systems. Springer-Verlag, Berlin
Bureau of Meteorology (2010) Climate statistics for Australian locations. Retrieved from http://www.bom.gov.au/climate/averages/tables/cw_009021.shtml in September, 2010
Busch DE, Ingraham NL, Smith SD (1992) Water uptake in woody riparian phreatophytes of the southeastern United States: a stable isotope study. Ecol Appl 2:450–459
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595
Canham CA, Froend RH, Stock WD (2009) Water stress vulnerability of four Banksia species in contrasting ecohydrological habitats on the Gnangara Mound, Western Australia. Plant Cell Environ 32:64–72
Castelli RM, Chambers JC, Tausch RJ (2000) Soil-plant relations along a water-table gradient in Great Basin riparian meadows. Wetlands 20:251–266
Cooper DJ, D’Amico DR, Scott ML (2003) Physiological and morphological response patterns of Populus deltoides to alluvial groundwater pumping. Environ Manage 31:215–226
Dawson TE, Pate JS (1996) Seasonal water uptake and movement in root systems of Australian phreatophytic plants of dimorphic root morphology: a stable isotope investigation. Oecologia 107:13–20
Dodd J, Bell DT (1993) Water relations of the canopy species in a Banksia woodland, Swan Coastal Plain, Western Australia. Aust J Ecol 18:281–293
Dodd J, Heddle EM, Pate JS, Dixon KW (1984) Rooting patterns of sandplain plants and their functional significance. In: Pate J, Beard J (eds) Kwongan plant life of the sand plain. University of Western Australia Press, Nedlands, pp 146–177
Eamus D, Hatton T, Cook P, Colvin C (2006) Ecohydrology: vegetation function, water and resource management. CSIRO Publishing, Collingwood
Farrington P, Greenwood EA, Bartle GA, Beresford JD, Watson GD (1989) Evaporation from Banksia woodland on a groundwater mound. J Hydrol 105:173–186
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall Inc., New Jersey
Gentilli J (1972) Australian Climate Patterns. Thomas Nelson, Melbourne
Greacen EL (1981) Soil water assessment by the neutron method. CSIRO (Division of Soils), Adelaide
Groom PK (2004) Rooting depth and plant water relations explain species distribution patterns within a sandplain landscape. Funct Plant Biol 31:423–428
Hendrick RL, Pregitzer KS (1997) The relationship between fine root demography and the soil environment in northern hardwood forests. Ecoscience 4:99–105
Horton JL, Clark J (2001) Water table decline alters growth and survival of Salix goodingii and Tamarix chinensis seedlings. Forest Ecol and Manag 140:239–247
Imada S, Yamanaka N, Tamai S (2008) Water table depth affects Populus alba fine root growth and whole plant biomass. Funct Ecol 22:1018–1026
Imada S, Yamanaka N, Tamai S (2010) Fine-root growth, fine-root mortality, and leaf morphological change of Populus alba in response to fluctuating water tables. Trees 24:499–506
Joslin JD, Wolfe MH, Hanson PJ (2001) Factors controlling the timing of root elongation intensity in a mature upland oak stand. Plant Soil 228:201–212
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiology Monograph 1:1–29
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press Inc., San Diego
Kranjcec J, Mahoney JM, Rood SB (1998) The responses of three riparian cottonwood species to water table decline. Forest Ecol Manag 110:77–87
Kummerow J, Krause D, Jow W (1978) Seasonal changes of fine root density in the southern Californian chaparral. Oecologia 37:201–212
Lamont BB (2003) Structure, ecology and physiology of root clusters—a review. Plant Soil 248:1–19
Lamont BB, Bergl SM (1991) Water relations, shoot and root architecture, and phenology of three co-occurring Banksia species: no evidence for niche differentiation in the pattern of water use. Oikos 60:291–298
Lubczynski MW (2009) The hydrogeological role of trees in water-limited environments. Hydrogeol J 17:247–259
Mahoney JM, Rood SB (1991) A device for studying the influence of declining water table on poplar growth and survival. Tree Physiol 8:305–314
Mahoney JM, Rood SB (1998) Streamflow requirements for cottonwood seedling recruitment—an integrative model. Wetlands 18:634–645
Martin D, Chambers J (2002) Restoration of riparian meadows degraded by livestock grazing: above and belowground responses. Plant Ecol 163:77–91
Meinzer OE (1923) Outline of groundwater hydrology with definitions. Water-supply paper 494. US Geological Survey
Montenegro G, Araya S, Aljaro ME, Avila G (1982) Seasonal fluctuations of vegetative growth in roots and shoots of Central Chilean shrubs. Oecologia 53:235–237
Murray BR, Zeppel MJ, Hose GC, Eamus D (2003) Groundwater dependent ecosystems in Australia: it’s more than just water for rivers. Ecol Manage Restor 4:110–113
Naumburg E, Mata-Gonzalez R, Hunter R, McLendon T, Martin D (2005) Phreatophytic vegetation and groundwater fluctuations: a review of current research and application of ecosystem response modelling with an emphasis on Great Basin vegetation. Environ Manage 35:726–740
Palacio S, Montserrat-Martí G (2007) Above and belowground phenology of four Mediterranean sub-shrubs: preliminary results on root-shoot competition. J Arid Environ 68:522–533
Poole DK, Miller PC (1975) Water relations of selected chapparal and coastal sage communities. Ecology 56:1118–1128
Popiel LO, Wojtkowiak J, Biernacka B (2001) Measurements of temperature distribution in ground. Exp Therm Fluid Sci 25:301–309
Purnell HM (1960) Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Aust J Bot 8:38–50
Richards MB, Stock WD, Cowling RM (1995) Water relations of seedlings and adults of two fynbos Proteaceae species in relation to their distribution patterns. Funct Ecol 9:575–583
Scott M, Shafroth P, Auble G (1999) Responses of riparian cottonwoods to alluvial water table declines. Environ Manage 23:347–358
Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Funct Ecol 16:367–378
Stave J, Oba G, Eriksen AB, Nordal I, Stenseth BC (2005) Seedling growth of Acacia tortilis and Faidherbia albida in response to simulated groundwater tables. Forest Ecol Manag 212:367–375
Steinaker DF, Wilson SD, Peltzer DA (2010) Asynchronicity in root and shoot phenology in grasses and woody plants. Global Change Biol 16:2241–2251
Teskey RO, Hinckley TM (1981) Influence of temperature and water potential on root growth of white oak. Physiol Plantarum 52:363–369
Veneklass EJ, Poot P (2003) Seasonal patterns in water use and leaf turnover of different plant functional types in a species rich woodland, south-western Australia. Plant Soil 257:295–304
Voroney RP (2007) The Soil Habitat. In: Eldor AP (ed) Soil microbiology and biochemistry, 3rd edn. Elsevier Inc., USA, pp 25–53
Zencich SJ, Froend RH, Turner JV, Gailitis V (2002) Influence of groundwater depth on the seasonal sources of water accessed by Banksia tree species on a shallow, sandy coastal aquifer. Oecologia 131:8–19
Acknowledgments
This research was conducted under an Australian Postgraduate Award associated with Australian Research Council Linkage Project LP0669240. The authors also wish to acknowledge the support of the Water Corporation of Western Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Rights and permissions
About this article
Cite this article
Canham, C.A., Froend, R.H., Stock, W.D. et al. Dynamics of phreatophyte root growth relative to a seasonally fluctuating water table in a Mediterranean-type environment. Oecologia 170, 909–916 (2012). https://doi.org/10.1007/s00442-012-2381-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2381-1