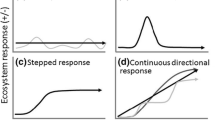
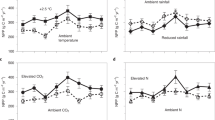
Abstract
Over the past 25 years, countless experiments have been conducted on the impact of increased atmospheric CO2 concentration on various plants and ecosystems. While this research was motivated to better understand and predict how rising CO2 will affect the structure and function of ecosystems in the future, it also shed light on some general, CO2-research independent, aspects in ecological research. Interestingly, it is these general aspects that continue to create confusion and lead to misinterpretation. Here, we focus on seven interrelated key issues including (1) the confusion between fluxes and pools, (2) the stoichiometric aspects of growth and biomass production, (3) resource allocation within organisms, (4) data scaling and the choice of a reference metric, (5) the consideration of time and timing (experimental duration, ontogenetic shifts), (6) confounding and second-order (indirect or feedback) effects, and (7) the key role of biodiversity. The principles deriving from addressing these issues relate strongly to each other. Their concurrent consideration requires experimenters and modellers to likewise maintain a broad, holistic perspective. In this synthesis, we attempt to show how appropriate consideration of these principles can greatly enhance the assessment of the validity, plausibility and generality of experimental and modelling results. We conclude that neglecting to adequately address these key issues in ecological research may lead to overestimations of measured responses and/or simplistic interpretations. Our examples mostly originate from research on plant responses to elevated atmospheric CO2, but are also applicable to other areas of ecological research. We provide a checklist for the planning of ecological field experiments and the interpretation of their results that may help in avoiding common pitfalls.

Similar content being viewed by others
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO(2) enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol 165:351–371
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Arnone JA, Körner C (1995) Soil and biomass carbon pools in model communities of tropical plants under elevated CO2. Oecologia 104:61–71
Asshoff R, Zotz G, Körner C (2006) Growth and phenology of mature temperate forest trees in elevated CO2. Glob Change Biol 12:848–861
Bazzaz FA, Grace J (1997) Plant resource allocation. Academic, San Diego
Bernal S, Hedin LO, Likens GE, Gerber S, Buso DC (2012) Complex response of the forest nitrogen cycle to climate change. Proc Natl Acad Sci USA 109:3406–3411
Bloom AJ, Chapin FS III, Mooney HA (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16:363–392
Boisvenue C, Running SW (2006) Impacts of climate change on natural forest productivity—evidence since the middle of the 20th century. Glob Change Biol 12:862–882
Bolker BM, Pacala SW, Bazzaz FA, Canham CD, Levin SA (1995) Species-diversity and ecosystem response to carbon-dioxide fertilization—conclusions from a temperate forest model. Glob Change Biol 1:373–381
Brouwer R (1962) Distribution of dry matter in the plant. Netherlands. J Agric Sci 10:361–376
Callaway RM, DeLucia EH, Thomas EM, Schlesinger WH (1994) Compensatory responses of CO2 exchange and biomass allocation and their effects on the relative growth-rate of ponderosa pine in different CO2 and temperature regimes. Oecologia 98:159–166
Ceulemans R, Mousseau M (1994) Tansley Review No-71—effects of elevated atmospheric CO2 on woody-plants. New Phytol 127:425–446
Chapin FS III, McFarland J, McGuire AD, Euskirchen ES, Ruess RW, Kielland K (2009) The changing global carbon cycle: linking plant-soil carbon dynamics to global consequences. J Ecol 97:840–850
Clark DA, Clark DB (1992) Life-history diversity of canopy and emergent trees in a neotropical rain-forest. Ecol Monogr 62:315–344
Cohen L (1988) Everybody Knows. I’m Your Man. Sony Music Entertainment (Canada) Inc., Toronto
Cramer W et al (2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob Change Biol 7:357–373
Curtis PS, Wang XZ (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
de Graaff M-A, van Groenigen K-J, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–2091
Drake JE et al (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO(2). Ecol Lett 14:349–357
Elser JJ et al (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Erice G, Sanz-Sáez A, Aranjuelo I, Irigoyen JJ, Aguirreolea J, Avice J-C, Sánchez-Díaz M (2011) Photosynthesis, N2 fixation and taproot reserves during the cutting regrowth cycle of alfalfa under elevated CO2 and temperature. J Plant Physiol 168:2007–2014
Feng XH (1999) Trends in intrinsic water-use efficiency of natural trees for the past 100–200 years: a response to atmospheric CO2 concentration. Geochim Cosmochim Acta 63:1891–1903
Field CB, Jackson RB, Mooney HA (1995) Stomatal responses to increased CO2: implications from the plant to the global scale. Plant Cell Environ 18:1214–1225
Finzi AC, Moore DJP, DeLucia EH, Lichter J, Hofmockel KS, Jackson RB, Hyun-Seok K, Matamala R, McCarthy HR, Oren R, Pippen JS, Schlesinger WH (2006) Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87:15–25
Friend AD (2010) Terrestrial plant production and climate change. J Exp Bot 61:1293–1309
Gedroc JJ, McConnaughay KDM, Coleman JS (1996) Plasticity in root shoot partitioning: optimal, ontogenetic, or both? Funct Ecol 10:44–50
Gifford RM, Evans LT (1981) Photosynthesis, carbon partitioning, and yield. Annu Rev Plant Physiol Plant Mol Biol 32:485–509
Granados J, Körner C (2002) In deep shade, elevated CO2 increases the vigor of tropical climbing plants. Glob Change Biol 8:1109–1117
Hagedorn F, van Hees PAW, Handa IT, Hättenschwiler S (2008) Elevated CO2 fuels leaching of old dissolved organic matter at the alpine treeline. Glob Biogeochem Cycles 22:GB2004. doi:10.1029/2007GB003026
Hagedorn F, Martin M, Rixen C, Rusch S, Bebi P, Zürcher A, Siegwolf RTW, Wipf S, Escape C, Roy J, Hättenschwiler S (2010) Short-term responses of ecosystem carbon fluxes to experimental soil warming at the Swiss alpine treeline. Biogeochemistry 97:7–19
Handa IT, Körner C, Hattenschwiler S (2005) A test of the tree-line carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology 86:1288–1300
Handa IT, Körner C, Hättenschwiler S (2006) Conifer stem growth at the altitudinal treeline in response to four years of CO2 enrichment. Glob Change Biol 12:2417–2430
Hättenschwiler S, Körner C (1996) System-level adjustments to elevated CO2 in model spruce ecosystems. Glob Change Biol 2:377–387
Hättenschwiler S, Körner C (1998) Biomass allocation and canopy development in spruce model ecosystems under elevated CO2 and increased N deposition. Oecologia 113:104–114
Hättenschwiler S, Körner C (2000) Tree seedling responses to in situ CO2-enrichment differ among species and depend on understorey light availability. Glob Change Biol 6:213–226
Hättenschwiler S, Körner C (2003) Does elevated CO2 facilitate naturalization of the non-indigenous Prunus laurocerasus in Swiss temperate forests? Funct Ecol 17:778–785
Hättenschwiler S, Miglietta F, Raschi A, Körner C (1997a) Morphological adjustments of mature Quercus ilex trees to elevated CO2. Acta Oecol 18:361–365
Hättenschwiler S, Miglietta F, Raschi A, Körner C (1997b) Thirty years of in situ tree growth under elevated CO2: a model for future forest responses? Glob Change Biol 3:463–471
Hoch G, Körner C (2003) The carbon charging of pines at the climatic treeline: a global comparison. Oecologia 135:10–21
Hoch G, Körner C (2012) Global patterns of mobile carbon stores in trees at the high-elevation tree line. Glob Ecol Biogeogr 21:861–871
Hoch G, Popp M, Körner C (2002) Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 98:361–374
Holtum JAM, Winter K (2010) Elevated CO2 and forest vegetation: more a water issue than a carbon issue? Funct Plant Biol 37:694–702
Idso SB, Kimball BA (1993) Tree growth in carbon-dioxide enriched air and its implications for global carbon cycling and maximum levels of atmospheric CO2. Glob Biogeochem Cycles 7:537–555
IPCC (2007) Climate change 2007: fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Iversen CM, Ledford J, Norby RJ (2008) CO(2) enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol 179:837–847
Iversen CM, Hooker TD, Classen AT, Norby RJ (2011) Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated CO(2). Glob Change Biol 17:1130–1139
Jackson RB, Cook CW, Pippen JS, Palmer SM (2009) Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90:3352–3366
Jacobs CMJ, de Bruin HAR (1997) Predicting regional transpiration at elevated atmospheric CO2: influence of the PBL-vegetation interaction. J Appl Meteorol 36:1663–1675
Jasoni RL, Smith SD, Arnone JA (2005) Net ecosystem CO2 exchange in Mojave Desert shrublands during the eighth year of exposure to elevated CO2. Glob Change Biol 11:749–756
Keel S, Siegwolf RTW, Körner C (2006) Canopy CO2 enrichment permits tracing the fate of recently assimilated carbon in a mature deciduous forest. New Phytol 172:319–329
Kimball BA, Mauney JR, Nakayama FS, Idso SB (1993) Effects of increasing atmospheric CO2 on vegetation. Vegetation 104:65–75
Kimball BA, Idso SB, Johnson S, Rillig MC (2007) Seventeen years of carbon dioxide enrichment of sour orange trees: final results. Glob Change Biol 13:2171–2183
King JS, Hanson PJ, Bernhardt E, DeAngelis P, Norby RJ, Pregitzer KS (2004) A multiyear synthesis of soil respiration responses to elevated atmospheric CO2 from four forest FACE experiments. Glob Change Biol 10:1027–1042
Knapp PA, Soule PT (2011) Increasing water-use efficiency and age-specific growth responses of old-growth ponderosa pine trees in the Northern Rockies. Glob Change Biol 17:631–641
Kobe RK, Pacala SW, Silander JA, Canham CD (1995) Juvenile tree survivorship as a component of shade tolerance. Ecol Appl 5:517–532
Körner C (1999) Alpine plants: stressed or adapted? In: Press MC, Scholes JD, Barker MG (eds) Physiological plant ecology. Blackwell, Oxford, pp 297–311
Körner C (2003a) Alpine plant life: functional plant ecology of high mountain ecosystems. Springer, Berlin
Körner C (2003b) Carbon limitation in trees. J Ecol 91:4–17
Körner C (2003c) Ecological impacts of atmospheric CO2 enrichment on terrestrial ecosystems. Philos Trans R Soc Lond A 361:2023–2041
Körner C (2003d) Slow in, rapid out—carbon flux studies and Kyoto targets. Science 300:1242–1243
Körner C (2006) Plant CO2 responses: an issue of definition, time and resource supply. New Phytol 172:393–411
Körner C (2009a) Biological Carbon sinks: turnover must not be confused with capital! Gaia-Ecol Perspect Sci Soc 18:288–293
Körner C (2009b) Responses of humid tropical trees to rising CO(2). Annu Rev Ecol Evol Syst 40:61–79
Körner C, Arnone JA (1992) Responses to elevated carbon dioxide in artificial tropical ecosystems. Science 257:1672–1675
Körner C et al (2005) Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 309:1360–1362
Körner C, Morgan J, Norby R (2007) Terrestrial ecosystems in a changing world. CO2 fertilization: when, where, how much?. Springer, Berlin
Körner C, Stöcklin J, Reuther-Thiebaud L, Pelaez-Riedl S (2008) Small differences in arrival time influence composition and productivity of plant communities. New Phytol 177:698–705
Langley JA, Megonigal JP (2010) Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 466:96–99
Langley JA, McKinley DC, Wolf AA, Hungate BA, Drake BG, Megonigal JP (2009) Priming depletes soil carbon and releases nitrogen in a scrub-oak ecosystem exposed to elevated CO2. Soil Biol Biochem 41:54–60
Lauber W, Körner C (1997) In situ stomatal responses to long-term CO2 enrichment in calcareous grassland plants. Acta Oecol 18:221–229
Le Quere C et al (2009) Trends in the sources and sinks of carbon dioxide. Nat Geosci 2:831–836
Leakey ADB, Lau JA (2012) Evolutionary context for understanding and manipulating plant responses to past, present, and future atmospheric [CO2]. Philos Trans R Soc Lond B 367:613–629
Leuzinger S, Zotz G, Asshoff R, Korner C (2005) Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol 25:641–650
Leuzinger S, Luo YQ, Beier C, Dieleman W, Vicca S, Korner C (2011) Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol Evol 26:236–241
Liberloo M et al (2006) Woody biomass production during the second rotation of a bio-energy Populus plantation increases in a future high CO2 world. Glob Change Biol 12:1094–1106
Luo YQ, Reynolds JF (1999) Validity of extrapolating field CO2 experiments to predict carbon sequestration in natural ecosystems. Ecology 80:1568–1583
Luo Y, Weng E (2011) Dynamic disequilibrium of the terrestrial carbon cycle under global change. Trends Ecol Evol 26:96–104
Luo Y et al (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
Luo YQ et al (2011) Coordinated approaches to quantify long-term ecosystem dynamics in response to global change. Glob Change Biol 17:843–854
Matthews R (2000) Storks Deliver Babies (p = 0.008). Teach Stat 22:36–38
Millard P, Sommerkorn M, Grelet G-A (2007) Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytol 175:11–28
Morford SL, Houlton BZ, Dahlgren RA (2011) Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock. Nature 477:U78–U88
Morgan JA, Pataki DE, Körner C, Clark H, Del Grosso SJ, Grünzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JP, Nowak RS, Parton WJ, Polley HW, Shaw MR (2004) Water relations in grassland and desert ecosystems exposed to elevated CO2. Oecologia 140:11–25
Muller B et al (2011) Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J Exp Bot 62:1715–1729
Murray MB, Leith ID, Jarvis PG (1996) The effect of long term CO2 enrichment on the growth, biomass partitioning and mineral nutrition of Sitka spruce (Picea sitchensis (Bong) Carr). Trees Struct Funct 10:393–402
Niklaus PA, Körner C (2004) Synthesis of a six-year study of calcareous grassland responses to in situ CO2 enrichment. Ecol Monogr 74:491–511
Niklaus PA, Spinnler D, Körner C (1998) Soil moisture dynamics of calcareous grassland under elevated CO2. Oecologia 117:201–208
Norby RJ (2010) ORNL net primary productivity data, Oak Ridge National Laboratory. In: Carbon Dioxide Information Analysis Center, U.S. Department of Energy. http://cdiac.ornl.gov
Norby RJ, Zak DR (2011) Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annu Rev Ecol Evol Syst 42(42):181–203
Norby RJ, Gunderson CA, Wullschleger SD, Oneill EG, McCracken MK (1992) Productivity and compensatory responses of yellow-poplar trees in elevated CO2. Nature 357:322–324
Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R (1999) Tree responses to rising CO2 in field experiments: implications for the future forest. Plant Cell Environ 22:683–714
Norby RJ, Hartz-Rubin JS, Verbrugge MJ (2003) Phenological responses in maple to experimental atmospheric warming and CO2 enrichment. Glob Change Biol 9:1792–1801
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG (2004) Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA 101:9689–9693
Norby RJ et al (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA 102:18052–18056
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO(2) enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci USA 107:19368–19373
Oberhuber W, Swidrak I, Pirkebner D, Gruber A (2011) Temporal dynamics of nonstructural carbohydrates and xylem growth in Pinus sylvestris exposed to drought. Can J For Res Rev Canadienne De Recherche Forestiere 41:1590–1597
Oren R et al (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472
Petit JR, Jouzel J, Raynaud D, Barkov NI, Barnola JM, Basile I, Bender M, Chappellaz J, Davis M, Delaygue G, Delmotte M, Kotlaykov VM, Legrand M, Lipenkov VY, Lorius C, Pepin L, Ritz C, Saltzman E, Stievenard M (1999) Nature 399:429–436
Polis GA (1999) Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86:3–15
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Pritchard SG, Strand AE, McCormack ML, Davis MA, Oren R (2008) Mycorrhizal and rhizomorph dynamics in a loblolly pine forest during 5 years of free-air-CO2-enrichment. Glob Change Biol 14:1252–1264
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Reich PB, Tjoelker MG, Machado J-L, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439:457–461
Reynolds JF, Thornley JHM (1982) A shoot: root partitioning model. Ann Bot 49:585–597
Ries L, Sisk TD (2004) A predictive model of edge effects. Ecology 85:2917–2926
Scheffer M et al (2006) Small habitat size and isolation can promote species richness: second-order effects on biodiversity in shallow lakes and ponds. Oikos 112:227–231
Schleppi P, Bucher-Wallin I, Hagedorn F, Koerner C (2012) Increased nitrate availability in the soil of a mixed mature temperate forest subjected to elevated CO2 concentration (canopy FACE). Glob Change Biol 18:757–768
Sherratt TN, Wilkinson DM (2010) Big questions in ecology and evolution. Oxford University Press, New York
Spinnler D, Egli P, Körner C (2002) Four-year growth dynamics of beech-spruce model ecosystems under CO2 enrichment on two different forest soils. Trees Struct Funct 16:423–436
Stöcklin J, Körner C (1999) Interactive effects of elevated CO2, P availability and legume presence on calcareous grassland: results of a glasshouse experiment. Funct Ecol 13:200–209
Taneva L, Pippen JS, Schlesinger WH, Gonzalez-Meler MA (2006) The turnover of carbon pools contributing to soil CO2 and soil respiration in a temperate forest exposed to elevated CO2 concentration. Glob Change Biol 12:983–994
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Tricker PJ et al (2009) Water use of a bioenergy plantation increases in a future high CO2 world. Biomass Bioenergy 33:200–208
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Voelker SL, Muzika R-M, Guyette RP, Stambaugh MC (2006) Historical CO2 growth enhancement declines with age in Quercus and Pinus. Ecol Monogr 76:549–564
Von Felten S, Hättenschwiler S, Saurer M, Siegwolf R (2007) Carbon allocation in shoots of alpine treeline conifers in a CO2 enriched environment. Trees Struct Funct 21:283–294
Wang X, Taub DR (2010) Interactive effects of elevated carbon dioxide and environmental stresses on root mass fraction in plants: a meta-analytical synthesis using pairwise techniques. Oecologia 163:1–11
Wardlaw IF (1990) Tansley review no 27—the control of carbon partitioning in plants. New Phytol 116:341–381
Wittwer SH (1983) Rising atmospheric CO2 and crop productivity. HortScience 18:667–673
Würth MKR, Winter K, Korner C (1998) Leaf carbohydrate responses to CO2 enrichment at the top of a tropical forest. Oecologia 116:18–25
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147:201–222
Acknowledgments
We thank an anonymous reviewer for helpful comments on the text. S.L. received funding from the FP7 project ‘ACQWA’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Russell Monson.
Rights and permissions
About this article
Cite this article
Leuzinger, S., Hättenschwiler, S. Beyond global change: lessons from 25 years of CO2 research. Oecologia 171, 639–651 (2013). https://doi.org/10.1007/s00442-012-2584-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2584-5