Abstract
Key message
Physiological and metabolic analysis showed the difference in drought tolerance and revealed the common and specific metabolic regulations in Salix sinopurpurea and Salix suchowensis.
Abstract
Willows (Salix spp.) are important woody plants as promising sources for sustainable and renewable biomass. However, drought is highly detrimental to their growth and development. Deciphering the adaptation mechanism of willows to drought will provide a theoretical basis for the cultivation of drought-tolerant varieties. In this work, we investigated the physiological and metabolic responses to drought stress in Salix sinopurpurea and Salix suchowensis. The drought experiment was conducted on clonal plants from cuttings of the two willow species under greenhouse conditions. S. sinopurpurea exhibited higher drought tolerance, as evidenced by lower growth reduction, and higher leaf relative water content, water use efficiency and net photosynthesis rate than those of S. suchowensis under the same drought conditions. Metabolic profiling identified 67 and 64 differentially accumulated metabolites in S. sinopurpurea and S. suchowensis, respectively. These metabolites function as compatible solutes and energy reserves and in antioxidant protection to defend against drought stress. Carbohydrate, amino acid and lipid metabolic pathways were central in the metabolic regulations of the drought response in the two willow species. The accumulation of aspartate, glutamate, serine, threonine, and sedoheptulose particularly in S. sinopurpurea might equip this species with drought tolerance. Meanwhile, phenylalanine and phytosterols were specifically inhibited in S. suchowensis, which might be associated with its susceptibility to drought stress. Taken together, these results provide a framework for better understanding the metabolic responses of willow to drought stress.
Similar content being viewed by others
Introduction
Willows are a highly diverse group of dioecious catkin-bearing trees and shrubs. They comprise approximately 330–500 species that are mainly distributed in the Northern Hemisphere (Argus 2007; Karp et al. 2011). In recent decades, willows together with poplars have been widely recognized as the trees of choice for intensive and short-rotation forestry to fulfill the increased demand for forest-derived biomass for energy production (Dickmann 2006; Isebrands and Richardson 2013; Karp et al. 2011; Volk et al. 2018). Because of its rapid propagation from hardwood cuttings, strong ability to resprout after multiple harvests, high yields in short rotation coppice cycles (approximately 3- to 5-year harvesting interval), and minimal requirement for fertilizer inputs, the shrub willow has been identified as important sustainable and renewable biomass sources for the generation of bioenergy, biofuels and bioproducts (Hanley and Karp 2014; Karp et al. 2011; Volk et al. 2018). Moreover, willows are also used for ornamental landscape, windbreak and sand fixation, stream bank stabilization, and phytoremediation, and they play crucial roles in rehabilitating degraded land, restoring forest landscapes and mitigating climate change (Dai et al. 2017; Isebrands and Richardson 2013; Jia et al. 2016). Nevertheless, willows require an adequate supply of water and usually grow in alluvial or riparian habitats. Thus, most willow species are susceptible to drought environments (Kuzovkina et al. 2008; Wikberg and Ögren 2004). Drought is highly detrimental to their survival, growth and production. Observed and model-simulated changes in global drought patterns have predicted that drought will be severe and widespread under global warming (Dai 2013). Therefore, investigation of the response mechanism to drought and improvement of drought tolerance of willows will promote their better adaptation to environmental stresses and improve their biomass production.
At present, a few studies have shed light on the morphological, physiological, biochemical, and transcriptional changes in the drought responses of willows (Bonosi et al. 2010; Jia et al. 2019; Pucholt et al. 2015; Ronnberg-Wastljung et al. 2005). Genotype-specific effects of drought on growth and leaf phenolics were found in Salix myrsinifolia and its hybrids with Salix myrsinites (Turtola et al. 2005; Turtola et al. 2006). Although S. myrsinifolia plantlets grow faster, they are more susceptible to drought stress than the hybrid plantlets (Turtola et al. 2006). Another study has reported that genotype but not drought stress affects the foliar phenolic concentrations and leaf-beetle resistance in ten F2 full-sibling hybrids from Salix viminalis and Salix dasyclados (Glynn et al. 2004). Two willow genotypes originating from a cross between S. viminalis and S. viminalis × S. schwerinii have been analyzed for their physiological and transcriptional responses to drought in leaves and roots. These two genotypes displayed more different transcriptional responses in the leaves than the roots, and a core set of candidate genes are identified in the drought response (Pucholt et al. 2015). Transcriptome analysis of S. psammophila under drought stress indicates that multiple stress-related processes are involved in the drought response, and two hub genes of magnesium-dependent phosphatase 1 and WRKY33 from a weighted gene coexpression network are confirmed to improve plant drought tolerance (Jia et al. 2019). Despite these advances, the molecular mechanism responsible for adaptations to drought in willow has not been comprehensively elucidated.
The plant response to drought is a complex and dynamic process involving multiple genes, proteins and metabolites. Metabolites (i.e., direct signatures of biochemical functions) can decipher the biochemical pathways involved in cellular processes. To reveal the drought-induced changes in metabolic activities and the biochemical composition, metabolic profiles have been investigated in Arabidopsis, maize, wheat and other plant species (Bowne et al. 2012; Skirycz et al. 2010; Yang et al. 2018). The reprogramming of metabolic networks during drought mainly contains the citric acid cycle (TCA cycle), glycolysis, sugar synthesis, carbohydrate and lipid metabolism, salicylate signaling pathways, and proline biosynthesis (Guo et al. 2018; Javier et al. 2015; Zhang et al. 2015). Soluble carbohydrates, amino acids, amines and polyols serve as osmolytes or compatible solutes to stabilize cellular proteins and structures and maintain cell turgor under drought stress (Sun et al. 2016; Ullah et al. 2017; Seki et al. 2007). Furthermore, the accumulation of these compatible solutes confers protection against oxidative damage by scavenging reactive oxygen species (ROS) to assist in re-establishing the cellular redox balance (Mata et al. 2016). These studies indicate that metabolomics is a powerful tool for elucidating how plants reconfigure their metabolism to maintain metabolic homeostasis and adapt to drought stress (Obata and Fernie 2012). Thus, further investigation of metabolic reprogramming will provide a new perspective on the drought response and defense mechanisms of willows.
Salix sinopurpurea C. Wang and Chang Y. Yang and Salix suchowensis W. C. Cheng are two native shrub willow species in China. S. sinopurpurea is mainly distributed in Northwest and North China. It can grow in nutrient-deficient and drought soil conditions and is regarded as a good tree species in relation to water and soil conservation. S. suchowensis is mainly distributed in East and Central China, and its genome sequencing has been completed (Dai et al. 2014). These two willow species play important roles in maintaining the ecological environment and providing potential biomass sources. Our preliminary research showed that the drought tolerance of these two species differed. Based on the difference in their geographical distributions and drought tolerance, we hypothesized that different drought responses and regulation mechanisms might exist in S. sinopurpurea and S. suchowensis. The aims of this study are to: (i) define the drought tolerance of the two willow species by investigating the effects of drought stress on their growth and physiological characteristics; (ii) explore common and specific metabolic responses (including differentially accumulated metabolites and involved metabolic pathways) in the two species to drought stress; and (iii) discuss the possible mechanism underlying willow drought tolerance or susceptibility. The results showed that S. sinopurpurea possessed higher drought tolerance than S. suchowensis, and both common and specific metabolic alterations existed in the two willow species. These results will facilitate attempts to elucidate the drought response mechanisms of willows and to breed drought-tolerant varieties through genetic manipulation.
Materials and methods
Plant materials and drought experiments
We collected twigs of S. sinopurpurea from one 2-year-old robust individual in Yangling in Shaanxi Province, and we collected twigs of S. suchowensis from one 2-year-old robust individual in Nanjing in Jiangsu Province. These twigs were cut into 15–20 cm cuttings, which were subsequently planted at the experimental base of the Chinese Academy of Forestry to obtain clones for each species. For drought experiments, 1-year-old twigs of S. sinopurpurea and S. suchowensis from the experimental base were cut into 15 cm cuttings, which were then planted in 2 L plastic pots containing loam soil (one cutting per pot). The clonal plants were incubated in a greenhouse (temperature of 22–25 °C; photoperiod of 16 h light/8 h dark). The plants were normally watered and provided with 200 mL of full-strength Hoagland solution every 2 weeks before commencing the drought treatment. After cultivating the plants normally for 6 weeks, plants with uniform growth were exposed to four different relative soil moisture content (RSMC) according to the treatment method for poplar (Li et al. 2011): well-watered control (RSMC with 70–75%); mild drought (RSMC with 50–55%); moderate drought (RSMC with 35–40%); severe drought (RSMC with 15–20%). All pots were weighed in the afternoon (16:00–17:00 p.m.) every day and water was replenished to maintain the four corresponding RSMC levels. The increased weight of the plants during drought treatment was omitted because it was dinky relative to the weight of soil in the pots. The drought treatments were maintained for one month at four RSMC levels. Twenty-four clonal plants of each species were subjected to drought treatment, with six plants at each RSMC level as six biological replicates.
Measurement of growth and physiological responses to drought stress
Before and after the drought experiment, plant growth was recorded by measuring the height and ground diameter of each plant with a ruler and vernier caliper, respectively. At the end of the drought experiment, the physiological responses to drought stress were detected in the two willow species. The photosynthetic characteristics of the sixth and seventh fully expanded mature leaves were measured at 09:00–11:00 a.m. using GFS-3000 portable photosynthesis system (Heinz Walz Gmbh, Effeltrich, Germany). Net photosynthetic rate (Pn) and transpiration rate (Tr) were measured under the following conditions: photosynthetic photon flux density, 1800 µmol m-2·s-1; relative air humidity, 55–65%; CO2 concentration, 400 ± 5 µmol mol-1; and leaf temperature, 28 °C. Water use efficiency (WUE) was calculated as the ratio of Pn and Tr. The leaf relative water content (LRWC) of the fifth fully expanded mature leaf was calculated with the equation: LRWC (%) = [(fresh weight - dry weight)/(turgid weight - dry weight)] × 100%. The turgid weight of leaves was measured after being submerged in distilled water for 24 h, and then the leaves were oven-dried at 85 °C for 48 h to determine the dry weight. The electric conductivity of the fourth fully expanded mature leaf was measured with DDS-307 conductivity meter (Lei Ci, China). The second and third fully expanded leaves were harvested, rapidly mixed and frozen in liquid nitrogen, and finally stored at - 80 °C for subsequent metabolite analysis.
Metabolite extraction and derivatization
Metabolites were extracted and derivatized according to previous studies (Wang et al. 2016) with some modifications. Approximately 100 mg of leaf powder was dissolved in 1.8 mL of methanol: chloroform: water (5:2:2, v/v/v) extract buffer, and internal standards of 3 µL of nonadecanoic acid (2.0 mg·mL-1) and 30 µL of ribitol (0.2 mg·mL-1) were then added to the mixture. The mixture was placed in a supersonic extractor for 30 min to facilitate metabolite extraction, and centrifuged for 10 min at 11,000 r·min-1. The supernatant was collected and stored at 4 °C, after which the precipitate was re-suspended in 1.0 mL of methanol: chloroform (1:1, v/v) buffer and centrifuged for 10 min at 11,000 r·min-1. The resulting supernatant was combined with the supernatant stored at 4 °C. Subsequently, 500 µL of distilled water was added to the combined supernatant, and the solution was centrifuged for 5 min at 5000 r·min-1 at 4 °C. Finally, 900 µL of the polar extract and 300 µL of the lipophilic extract were sucked out and dried using a nitrogen gas stream without heating. After the completion of metabolite extraction, 50 µL of methoxyamine hydrochloride (20 mg·mL-1) dissolved in pyridine was added to the dried extracts, followed by incubation for 1.5 h at 30 °C. Finally, 80 µL of N-methyl-N-trifluoroacetamide was added to the above solution, which was incubated for 30 min at 37 °C to complete derivatization.
GC–TOF/MS analysis
To reveal the metabolic responses of willow to different drought conditions, the metabolites of S. sinopurpurea and S. suchowensis were detected using Pegasus IV GC-TOF/MS system (LECO, St. Joseph, USA). The fused silica capillary column DB-5 MS (30 m long × 0.32 mm internal diameter × 0.25 µm film thickness) (J & W Scientific, Folsom, CA, USA) was used to separate metabolites. Metabolites were analyzed as described in a previous study (Wang et al. 2017). Briefly, the injection temperature was set at 280 °C; the MS transfer line was set at 250 °C; and the ion source was adjusted to 200 °C. The gas flow rate through the column was 1.5 mL·min-1.
Data processing and statistical analysis
The data of growth and physiological characteristics were analyzed using Statistical Package for the Social Sciences (SPSS) 17.0 program. Data were shown as means and standard error. Initial height and ground diameter were used as covariant. Two-way analysis of variance (ANOVA) was employed to test the effects of species, drought and their interaction effects on growth and physiological characteristics. Within each willow species, Duncan’s multiple range test was employed to determine differences among the four RSMC levels, and the differences were considered significant if P < 0.05. At each of the four RSMC levels, t test was used to detect significant differences between the two willow species.
The raw data of metabolome were processed using chromaTOF software (version 4.0) (LECO, St. Joseph, USA). Sample weights were normalized to minimize any discrepancies, and the data were normalized according to min–max normalization. The metabolic matrix was imported into SIMCA-P 12.0 software (Umetrics, Umeå, Sweden) to perform partial least square discriminant analysis (PLS-DA). The variable importance plot (VIP) values were exported from the covariance structures in the PLS-DA hyperspace, and potential differentially accumulated metabolites were extracted according to the VIP > 1. Additionally, the non-parametric Mann–Whitney test in SPSS 17.0 program was used to distinguish differentially accumulated metabolites (P < 0.05). The compounds were identified using the NIST standard mass spectral databases with similarity ≥ 80%. Hierarchical clustering analysis (HCA) of the differentially accumulated metabolites was performed using MeV 4.9 software (Saeed et al. 2003). To gain further insight into the metabolic pathways involved in willow responses to drought, we conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the differentially accumulated metabolites using MetaboAnalyst 3.0 software (Xia et al. 2015). Over-representation analysis by hypergeometric test and pathway topology analysis by relative-betweeness centrality were used to identify the most relevant metabolic pathways involved in drought responses.
Results
Differential growth and physiological responses to drought stress in two willow species
At the end of the drought experiment, the morphological characteristics were showed in Fig. 1a. The plants under drought stress exhibited reduced growth compared to well-watered plants. Analysis of variance (ANOVA) indicated that drought had significant influences on height and ground diameter (Supplementary Table S1). Although the height of S. sinopurpurea was significantly lower than that of S. suchowensis at each RSMC level, the decrease range of height from well-watered (control) condition to severe drought condition was less for S. sinopurpurea (from 96.56 to 58.88 cm; a reduction of 37.68 cm or 39.02%) than for S. suchowensis (from 116.03 to 64.72 cm; a reduction of 51.31 cm or 44.22%) (Fig. 1). The ground diameter of S. sinopurpurea was slightly lower than that of S. suchowensis under control and mild drought conditions, and it had no obvious differences between the two willow species under moderate and severe drought conditions. However, the decrease range of ground diameter from control condition to severe drought condition was also less for S. sinopurpurea (from 5.08 to 4.28 mm; a reduction of 0.80 mm or 15.75%) than for S. suchowensis (from 5.40 to 4.26 mm; a reduction of 1.14 mm or 21.21%) (Fig. 1).
Phenotypic and growth characteristics of two willow species S. sinopurpurea and S. suchowensis under drought stress. a Photograph of willow plants subjected to drought stress for one month at four relative soil moisture content (RSMC) levels: control condition, mild drought, moderate drought, severe drought; b comparison of the height of two willow species; c comparison of the ground diameter of two willow species. The values were means ± SE. Within each species, the different letters indicated significant difference at P < 0.05 level among the four RSMC levels according to Duncan’s multiple range test. ** and *** on the histogram of S. suchowensis indicated the significant difference at each of four RSMC levels when compared with S. sinopurpurea at P < 0.01 and P < 0.001 levels, respectively; and ns indicated non-significant difference
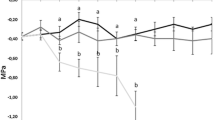
All physiological characteristics were significantly affected by species, drought and their interaction (Supplementary Table S1). The leaf relative water content (LRWC) and net photosynthesis rate (Pn) gradually decreased, while the relative electric conductivity (REC) and water use efficiency (WUE) increased as the extent of the drought increased (Fig. 2). Duncan’s multiple range test showed that the physiological characteristics had significant differences among four RSMC levels within each species, except for WUE of S. suchowensis between control and mild drought conditions (Fig. 2). No difference was detected in the four physiological characteristics between the two willow species under control condition (Fig. 2). Notably, S. sinopurpurea exhibited higher LRWC and WUE than S. suchowensis under the same drought stress (Fig. 2a, b). Although photosynthesis was inhibited by drought in the two willow species, S. sinopurpurea (12.34 µmol CO2·m-2·s-1 under control condition and 5.13 µmol CO2·m-2·s-1 under severe drought conditions; a reduction of 7.21 µmol CO2·m-2·s-1 or 58.43%) showed lower inhibition than S. suchowensis (12.46 µmol CO2·m-2·s-1 under control condition and 4.01 µmol CO2·m-2·s-1 under severe drought conditions; a reduction of 8.45 µmol CO2·m-2·s-1 or 67.82%) (Fig. 2c). Additionally, the REC, indicating membrane damage, was significantly lower for S. sinopurpurea than for S. suchowensis under moderate and severe drought conditions (Fig. 2d).
Comparison of the physiological characteristics of S. sinopurpurea and S. suchowensis under drought stress. a Leaf relative water content; b water use efficiency; c net photosynthetic rates; d leaf relative electrical conductivity. The values were means ± SE. Within each species, the different letters indicated significant difference at P < 0.05 level among the four RSMC levels according to Duncan’s multiple range test. *, ** and *** on the histogram of S. suchowensis indicated the significant difference at each of four RSMC levels when compared with S. sinopurpurea at P < 0.05, P < 0.01 and P < 0.001 levels, respectively; and ns indicated non-significant difference
Metabolite profiles of two willow species under drought stress
A total of 565 peaks, including 365 polar phases and 200 lipophilic phases, were identified for S. sinopurpurea. In S. suchowensis, 550 peaks were obtained, among which 358 and 192 peaks were identified in the polar and lipophilic phases, respectively. The score plots based on partial least squares discriminant analysis (PLS-DA) indicated that the metabolite profiles were well separated in both willow species (Fig. 3). The first principal component (PC1) was dominated by the two willow species, while the second principal component (PC2) was dominated by the four RSMC levels (Fig. 3). The trajectories of the drought-stressed samples were quite different in the two species: the metabolic changes in S. sinopurpurea occurred in a step-by-step manner from mild drought to severe drought, while the metabolite profiles of S. suchowensis under drought conditions were clustered together and separated from those of the control (Fig. 3).
Partial least squares discriminant analysis (PLS-DA) score plots of the metabolite profiles of the two willow species. Six biological replicates were conducted. The circle represented the metabolite profiles of S. sinopurpurea; the triangle represented the metabolite profiles of S. suchowensis. The ellipse represented the 95% confidence interval region according to Hotelling’s T2 statistic
Comparison of the metabolic responses of two willow species to drought stress
Compared with the control condition, differentially accumulated metabolites under mild, moderate, and severe drought conditions were screened using two criteria: VIP > 1.0 and Mann–Whitney P value < 0.05. In S. sinopurpurea, 67 differentially accumulated metabolites mainly including sugars, organic acids, amino acids, alcohols and amines were identified under drought conditions (Fig. 4a). Among these metabolites, the abundance of some metabolites was significantly increased under the three drought conditions, such as saccharic acid (7- to 18-fold), hexanoic acid (5- to 6-fold), galactose (4- to 6-fold), sedoheptulose (4- to 6-fold), lactose (3- to 6-fold) and hydroxycinnamic acid (3- to 4-fold), whereas the abundance of some metabolites was remarkably decreased, such as glycine (0.2- to 0.6-fold), succinate (0.3- to 0.5-fold) and malonic acid (0.3- to 0.5-fold). Meanwhile, 64 differentially accumulated metabolites were identified under drought conditions in S. suchowensis (Fig. 4b). The most highly increased metabolites were gluconic lactone (4- to 22-fold), saccharic acid (15- to 19-fold), lactose (19- to 33-fold), lyxose (4- to 7-fold), and mannose (3- to 10-fold), whereas the significantly decreased metabolites contained stigmasterol (0.38- to 0.69-fold), sitosterol (0.34- to 0.71-fold), oxirane (0.2- to 0.6-fold), and amiodarone (0.2- to 0.7-fold). Additionally, the metabolites (e.g., galactonic acid, glucose-1-phosphate, glycerol, glycine, oxirane and retinol) showed opposite changes between S. sinopurpurea and S. suchowensis (Fig. 4).
Hierarchical clustering analysis (HCA) of differentially accumulated metabolites in S. sinopurpurea and S. suchowensis under drought stress. a HCA of S. sinopurpurea; b HCA of S. suchowensis. The columns represented the three drought treatment groups (mild, moderate, and severe drought) compared with the control condition. The rows represented the metabolites. The colors corresponded to the log2-transformed values of fold changes in the three comparison groups. Increased and decreased levels of metabolites were indicated by increases in the intensities of yellow and blue, respectively.
Comparative analysis indicated that most of the differentially accumulated metabolites were overlapped in the three drought conditions, including 27 metabolites in S. sinopurpurea and 38 metabolites in S. suchowensis (Fig. 5). The number of differentially accumulated metabolites that overlapped between the moderate and severe drought conditions was more than the numbers identified in the other two comparison sets (mild versus moderate drought conditions, mild versus severe drought conditions), and the number of severe drought-specific differentially accumulated metabolites was larger than the numbers of mild drought-specific and moderate drought-specific metabolites. A total of 52 differentially accumulated metabolites were overlapped in S. sinopurpurea and S. suchowensis, albeit with different response modes and/or fold changes in some differentially accumulated metabolites in the two willow species (Figs. 4, 5c).
Venn diagrams of differentially accumulated metabolites showing common or uniquely regulated metabolites among the three comparison groups and between the two willow species. a Venn diagram for S. sinopurpurea; b Venn diagram for S. suchowensis; c Venn diagram for the two willow species. The black digits represented the whole number of differentially accumulated metabolites; the red and green digits represented the number of up-regulated and down-regulated metabolites, respectively.
Drought-induced regulation of carbohydrate metabolic pathways
Carbohydrates accounted for a large proportion of the differentially accumulated metabolites. Carbohydrate metabolism-associated pathways including galactose metabolism, starch and sucrose metabolism, ascorbate and aldarate metabolism, glycolysis/gluconeogenesis and TCA cycle were significantly enriched (p < 0.05) in S. sinopurpurea and S. suchowensis (Fig. 6). Metabolites involved in galactose metabolism (galactose, galactinol, raffinose, lactose, gluconic lactone, mannose, and myo-inositol) and starch and sucrose metabolism (sucrose, fructose, glucose, maltose, and trehalose) were significantly up-accumulated under drought conditions in the two species (Figs. 4, 7). In contrast, the abundance of TCA cycle intermediates (citrate, α-ketoglutarate, succinate, fumarate, and malate) decreased in both species in response to drought stress (Figs. 4, 7).
KEGG pathway analysis of differentially accumulated metabolites. a Metabolic pathways identified for S. sinopurpurea; b metabolic pathways identified for S. suchowensis. All the matched pathways were displayed with circles. X-axis represented the pathway impact values that were calculated by topology analysis, and the circle sizes corresponded to the pathway impact scores; Y-axis represented the -log(p) values that were estimated by over-representation analysis, and the circle colors corresponded to p values (darker colors indicated more significant changes in metabolites in the corresponding pathway). The significantly enriched pathways (p < 0.05) were annotated.
Comparison of metabolites involved in major metabolic pathways in S. sinopurpurea and S. suchowensis. The undetected metabolites were presented in gray. The solid and dashed arrows indicated single- and multi-step reactions, respectively. The grids located beside each metabolite represented the change levels under mild, moderate and severe drought conditions compared with the control condition. The colors corresponded to the log2-transformed values of fold changes in the three comparison groups.
Drought-induced regulation of amino acid metabolic pathways
The pathway of alanine, aspartate and glutamate metabolism was significantly enriched in S. sinopurpurea and S. suchowensis (Fig. 6). Metabolites such as alanine, lysine, proline and oxoproline were significantly up-accumulated under drought conditions, but asparagine was obviously down-regulated in the two species. In drought-tolerant S. sinopurpurea, another three pathways (glutathione metabolism, glycine, serine and threonine metabolism, and cyanoamino acid metabolism) were also involved in the metabolic response to drought, and some differentially accumulated metabolites (e.g., aspartate, glutamate, serine, and threonine) involved in these pathways were specifically increased in S. sinopurpurea (Figs. 4, 6, 7). Furthermore, we found that the abundance of phenylalanine only decreased in S. suchowensis and did not change in S. sinopurpurea (Fig. 4).
Drought-induced regulation of lipid metabolic pathways
Two lipid metabolic pathways including biosynthesis of unsaturated fatty acids and glycerolipid metabolism were significantly enriched in S. sinopurpurea and S. suchowensis (Fig. 6). Particularly, the abundance of four metabolites (palmitic acid, behenic acid, stearic acid, and α-linoleic acid) belonging to the biosynthesis pathway of unsaturated fatty acids increased obviously under drought conditions (Figs. 4, 7). Although glycerolipid metabolism was involved in both species, the drought responses of the metabolites (glycerol, glyceric acid, and glycerol-1-phosphate) were different. The abundance of glycerol increased in S. sinopurpurea but decreased in S. suchowensis. Glyceric acid exhibited reduced abundance, whereas glycerol-1-phosphate exhibited increased abundance in the two species under drought conditions (Fig. 4).
Discussion
Effect of drought stress in the growth and physiology of two willow species
In this study, we first compared the growth and physiological responses to different drought stresses in S. sinopurpurea and S. suchowensis. The height and ground diameter of S. suchowensis were higher than those of S. sinopurpurea under well-watered condition. Consistently with previous studies in some willow species and hybrids (Turtola et al. 2006; Fabio et al. 2019), the growth of S. sinopurpurea and S. suchowensis was significantly inhibited under drought condition. However, drought caused less growth reduction on S. sinopurpurea than S. suchowensis. Moreover, S. sinopurpurea showed higher LRWC, WUE and Pn than those of S. suchowensis under the same drought condition. LRWC and WUE are important physiological indices for evaluating plant adaption to drought, and the plants with high LRWC and WUE generally have strong drought tolerance (Steven 2011; Schittenhelm and Schroetter 2014; Yao et al. 2016). Photosynthesis is fundamental process for plant growth and development through converting the absorbed light energy into chemical energy (Petit et al. 2012; Melis 2013). Thus, our results demonstrated that S. sinopurpurea possessed higher drought tolerance than S. suchowensis. Furthermore, S. suchowensis had high growth rate under ideal growth condition, but its growth was severely diminished under drought stress, supporting the previous studies that fast-growing willow genotypes were most severely affected by drought stress (Weih 2001; Turtola et al. 2006; Fabio et al. 2019).
Overall metabolic responses of two willow species to drought stress
The metabolic profiles of the two species at four RSMC levels indicated that sugars, amino acids, organic acids, amines, fatty acids and alcohols were involved in the metabolic responses to drought stress. These differentially accumulated metabolites might act as compatible solutes or antioxidants, or play roles in the maintenance of membrane integrity to withstand drought stress. Common and specific metabolic alterations existed in the two willow species: (i) the metabolic pathways of carbohydrates, amino acids and lipids were involved in the common responses to drought in the two willow species; (ii) the accumulation of aspartate and glutamate involved in carbon/nitrogen balance specifically contributed to the drought tolerance of S. sinopurpurea; and (iii) phenylalanine serving as osmotic adjustment and phytosterols participating in the regulation of membrane permeability and fluidity were specifically inhibited in drought-susceptible S. suchowensis.
Common metabolic alterations in two willow species under drought stress
In this work, multiple carbohydrates were observed to significantly increase in S. sinopurpurea and S. suchowensis under drought conditions. Soluble carbohydrates exhibit the characteristics of high solubility, low molecular weight, no charge in physiological environment, rapid formation, and exerting little influence on metabolic activities and enzyme activities; thus, they are common osmotic regulators and play an essential role in scavenging ROS to protect against oxidative damage in plants (Guo et al. 2018; Li et al. 2017; Yang et al. 2018; Obata and Fernie 2012). In addition to primary carbohydrates such as glucose, sucrose, and fructose that contributed to the osmotic adjustment observed in many plants (Bowne et al. 2012; Guo et al. 2018; Tschaplinski et al. 2019), trehalose has been regarded as a potential mitigating agent against different abiotic stresses (Akram et al. 2016; Yatsyshyn et al. 2017). Its exogenous application can effectively improve the enzymatic and non-enzymatic antioxidant defense system in Raphanus sativus under drought stress (Shafiq et al. 2015), and overexpression of trehalose-6-phosphate phosphatase results in increased yields in maize under both the non-drought or mild drought conditions (Nuccio et al. 2015). The abundance of trehalose increased with the aggravation of drought stress in the two willow species, suggesting that trehalose might be associated with protection against drought damage. Additionally, as a versatile cellular compound, myo-inositol is the precursor of multiple compounds, such as pinitol, galactinol, raffinose family oligosaccharides and cell wall polysaccharides (Valluru and Ende 2011; Zhang et al. 2015). Among these compounds, the raffinose and galactinol play vital roles in protecting plants from cell dehydration and oxidative damage (Ayako et al. 2008; Barchet et al. 2013). The significant up-accumulation of myo-inositol, raffinose and galactinol in the two willow species might provide evidence of a close association between these metabolites and drought tolerance in plants. The accumulation of these carbohydrates could contribute to an increased cell osmotic potential, reduced cell dehydration rate, and facilitation of the maintenance of cell turgor pressure, thus mitigating the damage caused by drought to willow.
The abundance of ascorbate increased under drought conditions in S. sinopurpurea and S. suchowensis. Ascorbate is a ubiquitous water-soluble molecule serving as an antioxidant, a cofactor for enzymes, and a precursor for oxalate and tartrate synthesis (Smirnoff and Wheeler 2000). It participates in cell division and growth, photosynthesis, and resistance to environmental stresses (Smirnoff and Wheeler 2000; Hossain et al. 2013). Notably, ascorbate plays an important role to protect plant cells against the damaging effects of ROS, and it also plays a core role in ascorbate-glutathione cycle (Macknight et al. 2017). Enhancing the ascorbate content through biotechnological approaches has potential to improve plant tolerance to abiotic stresses. For example, overexpression of Medicago sativa GME (GDP-mannose 3, 5-epimerase) gene that involved in ascorbate biosynthesis can enhance drought and salt tolerance by increasing ascorbate in transgenic Arabidopsis (Ma et al. 2014). Co-expression of NCED1 (9-cis-Epoxycarotenoid dioxygenase) and ALO (D-arabinono-1,4-lactone oxidase) genes improves the levels of abscisic acid and ascorbate, and enhances tolerance to drought and chilling in transgenic Nicotiana tabacum and Stylosanthes guianensis plants (Bao et al. 2016). Furthermore, exogenous application of ascorbate in plants can alleviate the detrimental effects of salt and drought stresses through improving the antioxidative defense system and increasing the photosynthetic capacity and protein stability (Hossain et al. 2013; Noman et al. 2015). The increase of ascorbate under drought stress in the two willow species further suggested that the accumulation of ascorbate might be a critical defense strategy against drought in willow.
The significant accumulation of proline content was found in S. sinopurpurea and S. suchowensis under drought stress. Drought inhibits plant growth and disturbs nitrogen metabolism. During this process, decreased protein synthesis and enhanced proteolysis give rise to decreased protein content and simultaneous elevated amino acids (Ramanjulu and Sudhakar 1997). In willow, the most pronounced alteration was also found in amino acids, the majority of which displayed significant increase under drought conditions compared to well-watered condition. Proline is a known marker for drought stress, and its accumulation has been found to be an adaptive metabolic response to drought in many plants such as maize, wheat, Hordeum vulgare, Medicago truncatula and Astragalus membranaceus (Bowne et al. 2012; Jia et al. 2016; Hanna et al. 2017; Witt et al. 2012; Zhang et al. 2015). Proline has been commonly regarded as a vital drought-inducible metabolite because it plays crucial roles in dehydration avoidance through osmoregulation and desiccation tolerance through the maintenance of cellular redox homeostasis (Sharma et al. 2011; Szabados and Savouré 2010). The significant increase of proline in both two species indicated that it also played an essential role in the willow response to drought stress.
Accumulation of aspartate and glutamate might contribute to drought tolerance in S. sinopurpurea
The aspartate and glutamate increased significantly only in S. sinopurpurea, but they had no change in S. suchowensis. Aspartate and glutamate are central regulators of carbon/nitrogen metabolism (Less and Galili 2008; Mata et al. 2016). Carbon and nitrogen are the two most important elements required for normal plant growth and development. Their efficient acquisition and utilization are associated with increased crop productivity and quality. Only the carbon and nitrogen balance can maintain the normal growth of plants (Coruzzi and Zhou 2001). Thus, coordinating carbon and nitrogen metabolism enables plants to regulate metabolic responses to different environmental stimuli (Kang and FranK 2003). In addition, aspartate is the precursor of the essential amino acids such as lysine, threonine and isoleucine; while glutamate can be metabolized to γ-aminobutyrate, arginine and proline. These amino acids have generally been shown to accumulate under drought stress, especially in the drought-tolerant genotypes (Bowne et al. 2012; Mata et al. 2016; Yang et al. 2018); some of them have been demonstrated to contribute to the drought tolerance in plants (Joshi et al. 2010; Sharma et al. 2011; Yong et al. 2017). In this study, the accumulation of aspartate and glutamate in S. sinopurpurea implied that maintaining carbon and nitrogen balance and collaborating with their derived amino acids might be involved in the mechanisms underlying drought tolerance for S. sinopurpurea.
Decrease of phenylalanine and phytosterols might be associated with drought susceptibility in S. suchowensis
The phenylalanine decreased significantly only in S. suchowensis under dought stress. This result was in keeping with previous study showing reduction of phenylalanine by long-term drought stress in Cicer arietinum (Khan et al. 2019). Contrary finding has also indicated that phenylalanine increases in maize hybrids and lentil cultivars under drought stress (Witt et al. 2012; Muscolo et al. 2015). Phenylalanine is a critical metabolic node and it plays an essential role in the interconnection between primary and secondary metabolism in plants (Pascual et al. 2016). Phenylalanine is an important substrate in the phenylpropanoid pathway, through which numerous key secondary metabolites such as phenylpropanoids, flavonoids, and phenolic compounds are synthesized (Frelin et al. 2017; Yamada et al. 2008). These secondary metabolites play important roles to scavenge ROS to protect plants against from oxidative damage caused by abiotic stresses, including drought (Hernández et al. 2009; Velikova et al. 2016). The decrease of phenylalanine in S. suchowensis might inhibit the production of these protective metabolites and give rise to diminish the ability to resist drought, which might be associated with its susceptibility to drought stress.
The stigmasterol and sitosterol also decreased specifically in S. suchowensis under drought stress. Drought seriously impacts the plasma membrane of plant cells (Matos et al. 2010; Senthil-Kumar et al. 2013). Maintaining membrane integrity is crucial to confer drought tolerance in plants. Phytosterols are involved in regulation of membrane permeability and fluidity in addition to signal transduction for cell division and activity of membrane bound enzymes (Chen et al. 2018; Kumar et al. 2015). Stigmasterol, sitosterol, and campesterol are most common phytosterols, and each plant species own its characteristic phytosterol distribution (Kumar et al. 2015). Abundant stigmasterol incorporation into the plasma membrane can change its fluidity and permeability characteristics and limit the leakage of solutes from cytoplasm into apoplastic region (Senthil-Kumar et al. 2013). Some studies have provided evidence that fluctuation in the ratio of stigmasterol and sitosterol plays a role in modulating responses of plants to biotic and abiotic stresses (Arnqvist et al. 2008; Senthil-Kumar et al. 2013). The level of sitosterol has been found to be higher in drought tolerant rice seedlings than drought susceptible rice seedlings under drought stress (Kumar et al. 2015). In our study, the reduction of stigmasterol and sitosterol under drought stress in S. suchowensis suggested that S. suchowensis might exhibit a weak ability to maintain membrane integrity, which could be the reason that the REC indicating membrane damage was higher in S. suchowensis than in S. sinopurpurea.
Conclusions
In this work, we investigated the physiological and metabolic responses to drought stress in S. sinopurpurea and S. suchowensis. S. sinopurpurea exhibited higher drought tolerance, as evidenced by lower growth reduction and higher LRWC, WUE and Pn than those of S. suchowensis under the same drought conditions. The metabolic profiling analysis showed 67 and 64 metabolites were differentially accumulated in S. sinopurpurea and S. suchowensis, respectively. These metabolites mainly included sugars, organic acids, polyols, fatty acids, glycosides and amino acids. Common and specific metabolic responses existed in the two willow species. The carbohydrate, amino acid and lipid metabolic pathways were central in the drought responses of the two willow species. The accumulation of aspartate and glutamate involved in the cellular carbon/nitrogen balance might specifically contribute to the drought tolerance of S. sinopurpurea. The inhibition of phenylalanine and phytosterols in S. suchowensis might be associated with its susceptibility to drought stress. These results provide useful information for better understanding the mechanisms involved in willow drought tolerance.
Author Contribution statement
JHX and HJJ conceived and designed this study. JHX, WLJ, LJB and SP did the experiments. JHX and WLJ performed the data collation and statistical analysis. JHX, WLJ and LJB wrote the manuscript. LMZ and HJJ contributed with suggestions. All authors gave the final approval of the paper.
References
Akram NA, Waseem M, Ameen R et al (2016) Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: some key physio-biochemical traits. Acta Physiol Plant 38(3):1–10. https://doi.org/10.1007/s11738-015-2018-1
Argus GW (2007) Salix (Salicaceae) distribution maps and a synopsis of their classification in North America, North of Mexico. Harvard Pap Bot 12(2):335–368. https://doi.org/10.3100/1043-4534(2007)12[335:SSDMAA]2.0.CO;2
Arnqvist L, Persson M, Jonsson L et al (2008) Overexpression of CYP710A1 and CYP710A4 in transgenic Arabidopsis plants increases the level of stigmasterol at the expense of sitosterol. Planta 227(2):309–317. https://doi.org/10.1007/s00425-007-0618-8
Ayako N, Yukinori Y, Shigeru S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147:1251–1263. https://doi.org/10.1104/pp.108.122465
Bao G, Zhuo C, Qian C et al (2016) Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnol J 14:206–214. https://doi.org/10.1111/pbi.12374
Barchet GLH, Dauwe R, Guy RD et al (2013) Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol 34(11):1203–1219. https://doi.org/10.1093/treephys/tpt080
Bonosi L, Ghelardini L, Weih M (2010) Growth responses of 15 Salix genotypes to temporary water stress are different from the responses to permanent water shortage. Trees 24(5):843–854. https://doi.org/10.1007/s00468-010-0454-5
Bowne JB, Erwin TA, Juttner J et al (2012) Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol Plant 5(2):418–429. https://doi.org/10.1093/mp/ssr114
Chen M, Chen J, Luo N et al (2018) Cholesterol accumulation by suppression of SMT1 leads to dwarfism and improved drought tolerance in herbaceous plants. Plant Cell Environ 41(6):1417–1426. https://doi.org/10.1111/pce.13168
Coruzzi GM, Zhou L (2001) Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects’. Curr Opin Plant Biol 4(3):247–253. https://doi.org/10.1016/S1369-5266(00)00168-0
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58. https://doi.org/10.1038/nclimate1633
Dai X, Hu Q, Cai Q et al (2014) The willow genome and divergent evolution from poplar after the common genome duplication. Cell Res 24:1274–1277. https://doi.org/10.1038/cr.2014.83
Dai X, Zhu T, Li X et al (2017) Gene discovery and marker resource development by transcriptome sequencing from a short-rotation coppice willow, Salix suchowensis. Plant Breed 136:279–286. https://doi.org/10.1111/pbr.12458
Dickmann DI (2006) Silviculture and biology of short-rotation woody crops in temperate regions: then and now. Biomass Bioenerg 30(8–9):696–705. https://doi.org/10.1016/j.biombioe.2005.02.008
Fabio ES, Leary CJ, Smart LB (2019) Tolerance of novel inter-specific shrub willow hybrids to water stress. Trees 33(4):1015–1026. https://doi.org/10.1007/s00468-019-01835-4
Frelin O, Dervinis C, Wegrzyn JL et al (2017) Drought stress in Pinus taeda L. induces coordinated transcript accumulation of genes involved in the homogentisate pathway. Tree Genet Genomes 13:27. https://doi.org/10.1007/s11295-017-1115-2
Glynn C, Rönnberg-Wästljung AC, Julkunen-Tiitto R et al (2004) Willow genotype, but not drought treatment, affects foliar phenolic concentrations and leaf-beetle resistance. Entomol Exp Appl 113:1–14. https://doi.org/10.1111/j.0013-8703.2004.00199.x
Guo R, Shi L, Jiao Y et al (2018) Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. Aob Plants 10(2):1–13. https://doi.org/10.1093/aobpla/ply016
Hanley SJ, Karp A (2014) Genetic strategies for dissecting complex traits in biomass willows (Salix spp.). Tree Physiol 34(11):1167–1180. https://doi.org/10.1093/treephys/tpt089
Hanna B, Justyna N, Malgorzata P et al (2017) Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol Biochem 118:427–437. https://doi.org/10.1016/j.plaphy.2017.07.006
Hernández I, Alegre L, Breusegem FV et al (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14(3):125–132. https://doi.org/10.1016/j.tplants.2008.12.003
Hossain MA, Mostofa MG, Fujita M (2013) Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (Brassica campestris L.) seedlings. J Plant Sci Mol Breed 2050:1–14. https://doi.org/10.7243/2050-2389-2-2
Isebrands JG, Richardson J (2013) Introdution. In: Isebrands JG, Richardson J (eds) Poplars and willows: trees for society and the environment, 1st edn. CABI, Wallingford, pp 1–7. https://doi.org/10.1079/9781780641089.0000
Javier SM, Jim H, Alison KS et al (2015) A metabolomic study in oats (Avena sativa) highlights a drought tolerance mechanism based on salicylate signalling pathways and the modulation of carbon, antioxidant and photo-oxidative metabolism. Plant Cell Environ 38(7):1434–1452. https://doi.org/10.1111/pce.12501
Jia H, Yang H, Sun P et al (2016) De novo transcriptome assembly, development of EST-SSR markers and population genetic analyses for the desert biomass willow, Salix psammophila. Sci Rep 6:39591. https://doi.org/10.1038/srep39591
Jia H, Zhang J, Li J et al (2019) Genome-wide transcriptomic analysis of a desert willow, Salix psammophila, reveals the function of hub genes SpMDP1 and SpWRKY33 in drought tolerance. BMC Plant Biol 19:356. https://doi.org/10.1186/s12870-019-1900-1
Jia X, Sun C, Zuo Y et al (2016) Integrating transcriptomics and metabolomics to characterise the response of Astragalus membranaceus Bge. var. mongolicus (Bge.) to progressive drought stress. BMC Genom 17:188. https://doi.org/10.1186/s12864-016-2554-0
Joshi V, Joung JG, Fei Z et al (2010) Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids 39:933–947. https://doi.org/10.1007/s00726-010-0505-7
Kang J, Frank T (2003) The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc Natl Acad Sci USA 100(11):6872–6877. https://doi.org/10.1073/pnas.1030961100
Karp A, Steve HJ, Trybush SO et al (2011) Genetic improvement of willow for bioenergy and biofuels. J Integr Plant Biol 53(2):151–165. https://doi.org/10.1111/j.1744-7909.2010.01015.x
Khan N, Bano A, Rahman MA et al (2019) UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ 42(1):115–132. https://doi.org/10.1111/pce.13195
Kumar MS, Ali K, Dahuja A et al (2015) Role of phytosterols in drought stress tolerance in rice. Plant Physiol Biochem 96:83–89. https://doi.org/10.1016/j.plaphy.2015.07.014
Kuzovkina YA, Weih M, Romero MA et al (2008) Salix: botany and global horticulture. Hortic Rev 34:447–489. https://doi.org/10.1002/9780470380147.ch8
Less H, Galili G (2008) Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol 147:316–330. https://doi.org/10.1104/pp.108.115733
Li Z, Yu J, Peng Y et al (2017) Metabolic pathways regulated by abscisic acid, salicylic acid, and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol Plant 159(1):42–58. https://doi.org/10.1111/ppl.12483
Li B, Qin Y, Duan H et al (2011) Genome-wide characterization of new and drought stress responsive microRNAs in Populus euphratica. J Exp Bot 62(11):3765–3779. https://doi.org/10.1093/jxb/err051
Ma L, Wang Y, Liu W et al (2014) Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol Lett 36:2331–2341. https://doi.org/10.1007/s10529-014-1598-y
Macknight RC, Laing WA, Bulley SM et al (2017) Increasing ascorbate levels in crops to enhance human nutrition and plant abiotic stress tolerance. Curr Opin Biotechnol 44:153–160. https://doi.org/10.1016/j.copbio.2017.01.011
Mata AT, Jorge TF, Pires MV et al (2016) Drought stress tolerance in plants: insights from metabolomics. In: Hossain M, Wani S, Bhattacharjee S et al (eds) Drought stress tolerance in plants, 2ed. edn. Springer, Geneva, pp 187–216. https://doi.org/10.1007/978-3-319-32423-4_7
Melis A (2013) Carbon partitioning in photosynthesis. Curr Opin Chem Biol 17:453–456. https://doi.org/10.1016/j.cbpa.2013.03.010
Matos MC, Campos PS, Passarinho JA et al (2010) Drought effect on photosynthetic activity, osmolyte accumulation and membrane integrity of two Cicer arietinum genotypes. Photosynthetica 48(2):303–312. https://doi.org/10.1007/s11099-010-0038-z
Muscolo A, Junker A, Klukas C et al (2015) Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J Exp Bot 66(18):5467–5480. https://doi.org/10.1093/jxb/erv208
Noman A, Ali S, Naheed F et al (2015) Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch Agron Soil Sci 61(12):1659–1672. https://doi.org/10.1080/03650340.2015.1028379
Nuccio ML, Wu J, Mowers R et al (2015) Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33:862–869. https://doi.org/10.1038/nbt.3277
Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 69(19):3225–3243. https://doi.org/10.1007/s00018-012-1091-5
Pascual MB, El-Azaz J, Torre FN et al (2016) Biosynthesis and metabolic fate of phenylalanine in conifers. Front Plant Sci 7:1030. https://doi.org/10.3389/fpls.2016.01030
Petit AN, Fontaine F, Vatsa P et al (2012) Fungicide impacts on photosynthesis in crop plants. Photosynth Res 111:315. https://doi.org/10.1007/s11120-012-9719-8
Pucholt P, Sjödin P, Weih M et al (2015) Genome-wide transcriptional and physiological responses to drought stress in leaves and roots of two willow genotypes. BMC Plant Biol 15:244. https://doi.org/10.1186/s12870-015-0630-2
Ramanjulu S, Sudhakar C (1997) Drought tolerance is partly related to amino acid accumulation and ammonia assimilation: a comparative study in two mulberry genotypes differing in drought sensitivity. J Plant Physiol 150(3):345–350. https://doi.org/10.1016/S0176-1617(97)80131-9
Ronnberg-Wastljung AC, Glynn C, Weih M (2005) QTL analyses of drought tolerance and growth for a Salix dasyclados × Salix viminalis hybrid in contrasting water regimes. Theor Appl Genet 110(3):537–549. https://doi.org/10.1007/s00122-004-1866-7
Saeed A, Sharov V, White J et al (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34(2):374–378. https://doi.org/10.2144/03342mt01
Schittenhelm S, Schroetter S (2014) Comparison of drought tolerance of maize, sweet sorghum and sorghum-sudangrass hybrids. J Agro Crop Sci 200:46–53. https://doi.org/10.1111/jac.12039
Seki M, Umezawa T, Urano K et al (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10(3):296–302. https://doi.org/10.1016/j.pbi.2007.04.014
Senthil-Kumar M, Wang K, Mysore KS (2013) AtCYP710A1 gene-mediated stigmasterol production plays a role in imparting temperature stress tolerance in Arabidopsis thaliana. Plant Signal Behav 8:e23142. https://doi.org/10.4161/psb.23142
Shafiq S, Akram NA, Ashraf M (2015) Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci Hortic 185:68–75. https://doi.org/10.1016/j.scienta.2015.01.010
Sharma S, Villamor JG, Verslues PE (2011) Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol 157:292. https://doi.org/10.1104/pp.111.183210
Skirycz A, Bodt SD, Obata T et al (2010) Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol 152:226–244. https://doi.org/10.1104/pp.109.148965
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 19(4):291–314. https://doi.org/10.1080/07352680091139231
Steven JF (2011) Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol 190:249–257. https://doi.org/10.1111/j.1469-8137.2010.03603.x
Sun C, Gao X, Chen X et al (2016) Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric Water Manag 172:62–73. https://doi.org/10.1016/j.agwat.2016.04.016
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Tschaplinski TJ, Abraham PE, Jawdy SS et al (2019) The nature of the progression of drought stress drives differential metabolomic responses in Populus deltoides. Ann Bot 2:1–10. https://doi.org/10.1093/aob/mcz002
Turtola S, Rousi M, Pusenius J et al (2005) Clone-specific responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Global Chang Biol 11:1655–1663. https://doi.org/10.1111/j.1365-2486.2005.01013.x
Turtola S, Rousi M, Pusenius J et al (2006) Genotypic variation in drought response of willows grown under ambient and enhanced UV-B radiation. Environ Exp Bot 56(1):80–86. https://doi.org/10.1016/j.envexpbot.2005.01.007
Ullah N, Yuce M, Gokce ZNO et al (2017) Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom 18:969. https://doi.org/10.1186/s12864-017-4321-2
Valluru R, Ende WVD (2011) Myo-inositol and beyond-emerging networks under stress. Plant Sci 181(4):387–400. https://doi.org/10.1016/j.plantsci.2011.07.009
Velikova V, Brunetti C, Tattini M et al (2016) Physiological significance of isoprenoids and phenylpropanoids in drought response of Arundinoideae species with contrasting habitats and metabolism. Plant Cell Environ 39(10):2185–2197. https://doi.org/10.1111/pce.12785
Volk TA, Berguson B, Daly C et al (2018) Poplar and shrub willow energy crops in the United States: field trial results from the multiyear regional feedstock partnership and yield potential maps based on the PRISM-ELM model. GCB Bioenergy 10:735–751. https://doi.org/10.1111/gcbb.12498
Wang L, Qu L, Zhang L et al (2016) Metabolic responses of poplar to Apripona germari (Hope) as revealed by metabolite profiling. Int J Mol Sci 17(6):923. https://doi.org/10.3390/ijms17060923
Wang L, Qu L, Hu J et al (2017) Metabolomics reveals constitutive metabolites that contribute resistance to fall webworm (Hyphantria cunea) in Populus deltoides. Environ Exp Bot 136:31–40. https://doi.org/10.1016/j.envexpbot.2017.01.002
Weih M (2001) Evidence for increased sensitivity to nutrient and water stress in a fast-growing hybrid willow compared with a natural willow clone. Tree Physiol 21:1141–1148. https://doi.org/10.1093/treephys/21.15.1141
Wikberg J, Ögren E (2004) Interrelationships between water use and growth traits in biomass-producing willows. Trees 18(1):70–76. https://doi.org/10.1007/s00468-003-0282-y
Witt S, Galicia L, Lisec J et al (2012) Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol Plant 5(2):401–417. https://doi.org/10.1093/mp/ssr102
Xia J, Sinelnikov IV, Han B et al (2015) MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res 43(W1):W251–W257. https://doi.org/10.1093/nar/gkv380
Yamada T, Matsuda F, Kasai K et al (2008) Mutation of a rice gene encoding a phenylalanine biosynthetic enzyme results in accumulation of phenylalanine and tryptophan. Plant Cell 20(5):1316–1329. https://doi.org/10.1105/tpc.107.057455
Yang L, Fountain JC, Ji P et al (2018) Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol J 16(9):1616–1628. https://doi.org/10.1111/pbi.12899
Yao X, Yang R, Zhao F et al (2016) An analysis of physiological index of differences in drought tolerance of tomato rootstock seedlings. J Plant Biol 59:311–321. https://doi.org/10.1007/s12374-016-0071-y
Yatsyshyn YV, Kvasko YA, Yemets AI (2017) Genetic approaches in research on the role of trehalose in plants. Cytol Genet 51(5):371–383. https://doi.org/10.3103/S0095452717050127
Yong B, Xie H, Li Z et al (2017) Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol 8:1107. https://doi.org/10.3389/fphys.2017.01107
Zhang J, Carvalho MH, Torres-Jerez I et al (2015) Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ 37(11):2553–2576. https://doi.org/10.1111/pce.12328
Funding
This work was supported by National Nonprofit Institute Research Grant of CAF (CAFYBB2017ZY008) and (CAFYBB2018ZY001-9), and Natural Science Foundation of China (31800570).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no competing or financial interests.
Additional information
Communicated by Koike.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Analysis of variance (ANOVA) of growth and physiological characteristics of S. sinopurpurea and S. suchowensis under drought stress 1 (XLSX 11.5 kb)
Rights and permissions
About this article
Cite this article
Jia, H., Wang, L., Li, J. et al. Physiological and metabolic responses of Salix sinopurpurea and Salix suchowensis to drought stress. Trees 34, 563–577 (2020). https://doi.org/10.1007/s00468-019-01937-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-019-01937-z