Abstract
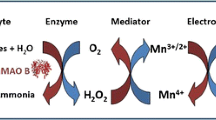
Electrochemical biosensors based on diamine oxidase (DAO) conjugated to magnetic beads (MBs) were developed for the detection of histamine (Hist), putrescine (Put) and cadaverine (Cad), the most relevant biogenic amines (BAs) related to food safety and quality. DAO-MBs were immobilised on Co(II)-phthalocyanine/carbon and Prussian Blue/carbon electrodes to obtain mono-enzymatic biosensors, and on Os-wired HRP-modified carbon electrodes to obtain bi-enzymatic biosensors. The three sensor have low working potentials (+0.4 V, −0.1 V and −0.05 V vs Ag/AgCl, respectively), a linear range of two orders of magnitude (from 0.01 to 1 mM BA), good reproducibility (variability lower than 10 %), high repeatability (up to 8 consecutive measurements), limits of detection in the µM concentration range for Hist and in the sub-µM concentration range for Put and Cad, and no response from possible interfering compounds. The DAO-MB conjugates display excellent long-term stability (at least 3 months). The biosensor has been applied to the determination of BAs in spiked and naturally-spoiled fish, demonstrating its suitability both as screening tool and for BAs quantification. The use of MBs as supports for enzyme immobilisation is advantageous because the resulting biosensors are simple, fast, stable, affordable, and can be integrated into array platforms. This makes them suitable for high-throughput analysis of BAs in the food industry.

Magnetic beads are used as diamine oxidase immobilisation supports for the development of amperometric biosensors for the detection of histamine, putrescine and cadaverine. Fast, low-cost, stable and easy-to-use biosensors for high-throughput monitoring of biogenic amines are achieved.
BA: Biogenic Amine; DAO: Diamine oxidase; HRP: Horseradish peroxidase.



Similar content being viewed by others
References
Santos MHS (1996) Biogenic amines: Their importance in foods. Int J Food Microbiol 29:213–231
Chong CY, Abu Bakar F, Russly AR, Jamilah B, Mahyudin NA (2011) The effects of food processing on biogenic amines formation. Int Food Res J 18:867–876
Stratton JE, Hutkins RW, Taylor SL (1991) Biogenic-Amines in Cheese and Other Fermented Foods - a Review. J Food Prot 54:460–470
Comission Regulation No. 1441/2007 of December 5, 2007. Off. J Eur Communities, L139 (2007) 11.82
Önal A (2007) A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem 103:1475–1486
Henao-Escobar W, Del Torno-de Roman L, Domínguez-Renedo O, Alonso-Lomillo MA, Arcos-Martínez MJ (2016) Dual enzymatic biosensor for simultaneous amperometric determination of histamine and putrescine. Food Chem 190:818–823
Henao-Escobar W, Domínguez-Renedo O, Alonso-Lomillo MA, Cascalheira JF, Dias-Cabral AC, Arcos-Martínez MJ (2015) Characterization of a disposable electrochemical biosensor based on putrescine oxidase from micrococcus rubens for the determination of putrescine. Electroanalysis 27:368–377
Henao-Escobar W, Domínguez-Renedo O, Alonso-Lomillo MA, Arcos-Martínez MJ (2013) Simultaneous determination of cadaverine and putrescine using a disposable monoamine oxidase based biosensor. Talanta 117:405–411
Henao-Escobar W, Domínguez-Renedo O, Alonso-Lomillo MA, Arcos-Martínez MJ (2013) A screen-printed disposable biosensor for selective determination of putrescine. Microchim Acta 180:687–693
Alonso-Lomillo MA, Domínguez-Renedo O, Matos P, Arcos-Martínez MJ (2010) Disposable biosensors for determination of biogenic amines. Anal Chim Acta 665:26–31
Pérez S, Bartrolí J, Fàbregas E (2013) Amperometric biosensor for the determination of histamine in fish samples. Food Chem 141:4066–4072
Telsnig D, Kassarnig V, Zapf C, Leitinger G, Kalcher K, Ortner A (2012) Characterization of an amperometric biosensor for the determination of biogenic amines in flow injection analysis. Int J Electrochem Sci 7:10476–10486
Telsnig D, Terzic A, Krenn T, Kassarnig V, Kalcher K, Ortner A (2012) Development of a voltammetric amine oxidase-modified biosensor for the determination of biogenic amines in food. Int J Electrochem Sci 7:6893–6903
Keow CM, Bakar FA, Salleh AB, Heng LY, Wagiran R, Siddiquee S (2012) Screen-printed histamine biosensors fabricated from the entrapment of diamine oxidase in a photocured poly(HEMA) film. Int J Electrochem Sci 7:4702–4715
Bóka B, Adányi N, Szamos J, Virág D, Kiss A (2012) Putrescine biosensor based on putrescine oxidase from Kocuria rosea. Enzym Microb Technol 51:258–262
Bóka B, Adányi N, Virág D, Sebela M, Kiss A (2011) Spoilage detection with biogenic amine biosensors, comparison of different enzyme electrodes. Electroanalysis 24:181–186
Di Fusco M, Federico R, Boffi A, Macone A, Favero G, Mazzei F (2011) Characterization and application of a diamine oxidase from Lathyrus sativus as component of an electrochemical biosensor for the determination of biogenic amines in wine and beer. Anal Bioanal Chem 401:707–716
Gumpu MB, Nesakumar N, Sethuraman S, Krishnan UM, Rayappan JBB (2014) Development of electrochemical biosensor with ceria-PANI core-shell nano-interface for the detection of histamine. Sensors Actuatots B Chem 199:330–338
Shanmugam S, Thandavan K, Gandhi S, Sethuraman S, Rayappan JBB, Krishnan UM (2011) Development and evaluation of a highly sensitive rapid response enzymatic nanointerfaced biosensor for detection of putrescine. Analyst 136:5234–5240
Yang X, Feng B, He X, Li F, Ding Y, Fei J (2013) Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim Acta 180:935–956
Reverté L, Prieto-Simón B, Campàs M (2016) New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidic systems. A review. Anal Chim Acta 908:8–21
Hasanzadeh M, Shadjou, la Guardia M D (2015) Iron and iron-oxide magnetic nanoparticles as signal-amplification elements in electrochemical biosensing. TrAC Trends Anal Chem 72:1–9
Pedrero M, Campuzano S, Pingarrón JM (2012) Magnetic beads-based electrochemical sensors applied to the detection and quantification of bioterrorism/biohazard agents. Electroanalysis 24:470–482
Shi X, Gu W, Li B, Chen N, Zhao K, Xian Y (2014) Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim Acta 181:1–22
Lindgren A, Ruzgas T, Gorton L, Csöregi E, Ardila GB, Sakharov IY, Gazaryan IG (2000) Biosensors based on novel peroxidases with improved properties in direct and mediated electron transfer. Biosens Bioelectron 15:491–4971
Gu M, Wang J, Tu Y, Di J (2010) Fabrication of reagentless glucose biosensors: A comparison of mono-enzyme GOD and bienzyme GOD-HRP systems. Sensors Actuators B Chem 148:486–491
Pospiskova K, Safarik I, Sebela M, Kuncová G (2013) Magnetic particles-based biosensor for biogenic amines using an optical oxygen sensor as a transducer. Microchim Acta 180:311–318
Garibo D, Devic E, Marty JL, Diogène J, Unzueta I, Blazquez M, Campàs M (2012) Conjugation of genetically engineered protein phosphatases to magnetic particles for okadaic acid detection. J Biotechnol 157:89–95
Shangavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182:1–41
Enache TA, Oliveira-Brett AM (2011) Phenol and para-substituted phenols electrochemical oxidation pathways. J Electroanal Chem 655:9–16
Acknowledgments
The authors acknowledge financial support from the Ministerio de Economía y Competitividad through the DIANA (BIO2011-26311) project. The authors also acknowledge Pere Campàs for the gift of sea bass samples. Sandra Leonardo acknowledges scholarship from IRTA – Universitat Rovira i Virgili – Banco Santander (2013PIPF URV-IRTA-BS-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 315 kb)
Rights and permissions
About this article
Cite this article
Leonardo, S., Campàs, M. Electrochemical enzyme sensor arrays for the detection of the biogenic amines histamine, putrescine and cadaverine using magnetic beads as immobilisation supports. Microchim Acta 183, 1881–1890 (2016). https://doi.org/10.1007/s00604-016-1821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1821-8