Abstract
Iridium nanoparticles (IrNPs) with intrinsic oxidase-like activity were synthesized by using sodium citrate as the stabilizer and NaBH4 as the reducing agent. The IrNPs have an average diameter of 2.5 ± 0.5 nm and exhibit excellent oxidase-like property. Under the catalytic action of the IrNPs, 3,3′,5,5′-tetramethylbenzidine (TMB) is oxidized by dissolved oxygen (DO) to form a blue product with an absorption maximum at 652 nm. The catalytic activity is ascribed to the production of superoxide anion radical (O2ˉ∙). The chromogenic reaction is exploited for the determination of DO. The method exhibits a wide calibration range from 12.5 to 257.5 μM of DO and a limit of detection as low as 4.7 μM. Compared to other methods, this method presented here shows improved precision and faster response time.

Iridium nanoparticles (IrNPs) stabilized by sodium citrate exhibit oxidase-like activity and can effectively catalyze dissolved oxygen (DO) by oxidizing 3,3′,5,5′-tetramethylbenzidine (TMB) to form a blue product.
Similar content being viewed by others
Introduction
Natural enzymes have been widely used in biosensing, pharmaceutical processes and food industry. Some intrinsic drawbacks, however, are often associated with natural enzymes, including time-consuming and expensive production, sophisticated purification, and low stability to temperature and pH variations [1, 2]. Enzyme mimics of nanomaterials have shown a number of advantages over the natural enzymes, such as their super catalytic performance and stability [3,4,5]. Especially the metal nanomaterials enclosed with high-index facet, possess high density of low-coordinated atoms. Hence they have more active sites and exhibit superb catalytic characteristics [6]. Many research efforts are devoted to the preparation of noble metal nanomaterials with high-index facets by controlling the surface atomic arrangement and configuration. For examples, Cu2O octahedral with high-index facets have been prepared. Their glucose oxidase and horseradish peroxidase-like activity have been explored [7].
Among various metal nanomaterials, IrNPs emerged as a potent catalyst and have attracted a great deal interests in the fields of the catalytic hydrogenation reaction, batteries and fuel cells [8, 9]. Their enzyme mimics property, however, were less frequently studied. The IrNPs capped with polyvinylpyrrolidone (PVP-IrNPs) can protect cells from H2O2-induced oxidative damage, suggesting that the PVP-IrNPs have peroxidase-like activity [10]. We also confirmed that the IrNPs stabilized by tannic acid (TA) showed the peroxidase-like activity. They can catalyze H2O2 to oxidize TMB forming blue oxTMB through electron transfer mechanism [11]. It was noted that the TMB can be oxidized even without the addition of peroxides, although long incubation time was required. The observation suggested that IrNPs may also possess oxidase activity. This can be a more important research topic and deserved further exploration.
Hence in this work, the polycrystalline IrNPs enclosed with the {200}, {220} and {311} high-index facets were prepared in one-pot synthesis using sodium citrate as stabilizer. The IrNPs exhibit super oxidase-like activity in the oxidation of TMB, hence they were further exploited for the determination of DO in water. Dissolved oxygen refers to the level of free, non-compound oxygen present in water. It is an important parameter in assessing water quality because of its influence on the organisms living within a body of water. Hence the determination of DO is very important in environmental monitoring and physiological and biological applications [12, 13]. Our experiment results proved that the color reaction of the TMB oxidation is a convenient colorimetric method for rapid and sensitive determination of DO.
Experimental section
Reagents and instruments
Iridium(III) chloride trihydrate (IrCl3·3H2O) and 3,3,5,5-tetramathylbenzidine (TMB) were purchased from Aladdin Industrial Co., Ltd. (Shanghai, China, http://www.chemcd.com/supplier/aladdin.html). Horseradish peroxidase (HRP) was obtained from SangonBiotech Co., Ltd. (Shanghai, China, http://www.sangon.com/product). 1,3-diphenylisobenzofuran (DPBF), benzoquinone, ascorbic acid (Vc), trisodium citrate dihydrate (Na3C6H5O7·2H2O), tannic acid, polyvinylpyrrolidone (PVP), sodium borohydride (NaBH4), sodium hydroxide (NaOH), acetic acid (HAc), sodium acetate anhydrous (NaAc) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://shreagent.lookchem.com/). All reagents were of analytical grade and were used without further purification. Milli-Q ultrapure water (18.2 MΩ·cm) was used throughout all experiments.
Absorption spectra and kinetic assay were carried out on a UV-vis spectrophotometer with a 1 cm quartz cuvette (UV-2700, Japan Shimadzu Co., Ltd., http://www.shimadzu.com/an/molecular_spectro/uv/uv2600_2700.html). The fluorescence measurements were performed on a fluorescence spectrophotometer (Edinburgh Instruments, http://www.labwrench.com/?equipment.view/equipmentNo/18195/Edinburgh-Instruments/FS5/) with a 1.0 cm quartz cell (Ex slit 5 nm, Em slit 5 nm). High resolution transmission electron microscopy (HRTEM, JEM-2100, Japan electron optics laboratory co., Ltd., https://www.jeol.co.jp/en/products/detail/JEM-2100.html) was operated at 200 kV to visualize the prepared IrNPs. The sample for TEM measurement was prepared by slow evaporation of the IrNPs on a carbon film supported by 300-mesh Cu grids. Phase identification of the IrNPs were conducted with X-ray diffraction (XRD, D8, Bruker AXS Co., Ltd., http://www.vincent.org.rs/en/equipment/xrd-diffractometer-bruker-axs-d8-bruker-germany/) using Cu-Kα radiation source (λ = 1.54051 Å) over the 2θ range of 3-90o. The concentration of iridium was measured using inductively coupled plasma-mass spectrometry (ICP-MS, Agilent 7500, USA, http://snri.ucmerced.edu/environmental-analytical-laboratory/icp-ms).
Preparation of the IrNPs
IrNPs were prepared according to our previous paper [14]. Briefly, sodium citrate solution (3 mL, 0.034 M) was mixed with iridium chloride solution (20 mL, 1 mM). NaOH solution (1 M) was added dropwise to adjust the pH of mixture to 7–9. The mixture was refluxed under vigorous stirring for 20 min and the color of solution changed to faint yellow. Freshly prepared NaBH4 solution (1 mL, 0.1 M) was added and the mixture was stirred for 30 min with the heater switched off. To prevent the influence from oxygen, the whole preparation process was carried out under nitrogen atmosphere. The color of the solution changed gradually to black, indicating the formation of colloidal IrNPs. The colloidal IrNPs were purified by adding the appropriate amount of ethanol and centrifuging the mixture at 3357 rcf for 5 min. Washing the precipitate for three times by ethanol, and the obtained product was dried in vacuum at 45 °C. A stock solution (1.5 mg·mL−1) was prepared by dissolving the powders in ultrapure water and stored at room temperature for subsequent use. The concentration of Ir was measured to be 4.94 mM according to the ICP-MS results.
Kinetic analysis and catalytic mechanism study
The Michaelis-Menten behavior of the catalytic reactions was investigated by monitoring the absorbance of TMB at 652 nm. The experiments were carried out at room temperature with different concentrations of TMB in NaAc buffer (20 mM, pH 3.5) in presence of 17.23 nM of IrNPs. The concentration of DO in water was measured to be 257.5 μM by Winkler titration. Lineweaver-Burk plots, 1/v = (K m /V max )(1/[S]) + 1/V max , was used to calculate the Michaelis-Menten constant [15], where v represents the initial velocity, V max stands for the maximal reaction velocity, [S] is the concentration of substrate and K m is the Michaelis constant. The involvement of the reactive oxygen species (ROS) was investigated by recording the fluorescence spectra of DPBF in the presence or absence of IrNPs. The absorbance spectra of TMB in the presence and absence of IrNPs and benzoquinone were also recorded to identify the ROS categories in the reactions.
Determination of DO
A caped quartz cuvette with seal gasket was used for the preparation of solutions with different DO concentrations (Scheme S1). Firstly, a centrifugal tube was fixed to the cap with a piece of wire. An appropriate amount of TMB (0.45, 0.95, 1.20, 1.45, 1.50, 1.60, 1.66, 1.75, 1.77 and 1.85 mL in NaAc buffer) and 50 μL IrNPs (1.38 μM) solution were added into the quartz cuvette and centrifugal tube, respectively. The quartz cuvette was sealed with the cap to prevent the influence of oxygen from air. To establish an inert atmosphere, Schlenk line technique was used to bubble N2 through syringe needles a and b, and remove air through the needle c. Then an appropriate amount of water (1.50, 1.00, 0.75, 0.50, 0.45, 0.35, 0.29, 0.20, 0.18 and 0.10 mL) with 257.5 μM DO was injected to regulate the DO concentration of the reaction system. Finally, the centrifugal tube was pierced by needle b and the IrNPs solution was thus added to the TMB solution within the cuvette. The TMB was oxidized rapidly to form blue oxTMB and the UV-vis absorbance spectra were recorded immediately.
Sample analysis
Ethylenediaminetetraacetic acid (EDTA, 0.029 g) were added to 50 mL tap water, bottled water and lake water to eliminate the inference of metal ions. Then an aliquot of 200 μL NaAc buffer (pH 3.5, 1 mM), 100 μL TMB (25 mM) and 100 μL IrNPs (3.45 μM) were successively added to 9.6 mL water samples. The UV-vis absorbance spectra were recorded in 60 s and their DO concentrations were calculated.
Results and discussion
Characterization of the IrNPs
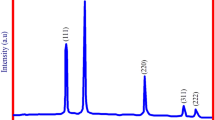
The IrNPs exhibit good monodispersity and the average diameter is about 2.5 ± 0.5 nm based on the statistic result of 108 particles (Fig. S1). The morphology can be readily observed in the HRTEM images, and crystal faces {111}, {200}, {220}, {311} are clearly resolved (Fig. 1a). The electron diffraction pattern of selected area shows that the IrNPs are composed of polycrystalline and reflections corresponded to {111}, {200}, {220}, {311} facets of iridium (Fig. 1b). XRD patterns indicate the 2θ values of these facets correspond to 40.9, 47.2, 69.1, and 83.6 degrees, respectively (Fig. 1c) [9]. Compared with the basal facets ({100}, {110}, {111}), high-index facets {200}, {220}, {311} endow the IrNPs with higher catalytic activity [16, 17].
Steady-state kinetic assay of the IrNPs catalytic reaction
In the presence of the IrNPs, TMB can be oxidized by DO to produce blue oxTMB and shows an absorption peak at 652 nm. While DO alone can not initiate the corresponding color reaction (Fig. 2a, b). The absorbance increases steadily with the increase of reaction time in the presence of IrNPs and DO. Whereas the absorbance remains constant in the absence of the IrNPs, suggesting that the IrNPs possess oxidase-like activity (Fig. 2c).Visible light has no effect on the oxidase-like activity of the IrNPs, thus we rule out the possible involvement of light induced catalysis (Fig. S2).
a Schematic illustration of color reaction of TMB oxidized by DO under the catalytic action of the IrNPs; b UV-vis absorbance spectra and c time-dependent absorbance of different reaction systems at 652 nm: (a) TMB + DO, (b) IrNPs + DO and (c) TMB + IrNPs + DO. Insert is the digital photos of color reaction of three systems. Reaction conditions: 250 μM TMB, 17.23 nM IrNPs, 257.5 μM DO, 20 mM NaAc buffer (pH 3.5), room temperature
The catalytic activity of nanomaterials is closely related to their stabilizer [17]. To investigate the effect of stabilizers, the catalytic rates in the presence of IrNPs stabilized respectively by PVP, tannic acid and citrate were compared and the results are presented in Fig. S3. As can be seen that the best oxidase-like activity is obtained from the IrNP stabilized by citrate, presumably due to the IrNPs stabilized by small molecule can expose more active sites on the surface of nanoparticles.
The IrNPs exhibit the optimal activity at pH 3.5 (Fig. S4A). The slightly acidic medium can speed up the oxidation rate due to the requirement of hydrogen ion in the reaction (Scheme S2) [18]. Moreover, the ionization of amino groups in TMB is pH-dependent, the proton concentration also influences the chemical reactivity of TMB [14]. The rate of color formation is almost independent of the temperature in the range of 20–35 °C. When the temperature is above 35 °C, the rate of color formation is slow down, presumably due to the decrease in DO concentration in elevated temperature (Fig. S4B). Thus, room temperature is selected as the reaction temperature in subsequent experiments.
TMB oxidation reaction was evaluated by steady-state kinetics analysis. Under the catalytic action of the IrNPs, a series of initial reaction rates were obtained by recording the time-dependent absorbance of different TMB concentrations. As shown in Fig. S5A, the TMB oxidation reaction follows the typical Michaelis-Menten behavior. The steady-state kinetic parameters K m and V max were obtained through fitting the initial reaction rates to the Michaelis-Menten equation using Lineweaver-Burk plots (Fig. S5B). The K m and V max of the IrNPs for TMB oxidation were calculated to be 280 μM and 0.1365 μM·s−1, respectively.
As shown in Table 1, the K m value of the Ir NPs with TMB is lower than HRP [11] and AuNPs [19], suggesting a higher affinity of the IrNPs to TMB than that of HRP and AuNPs. Due to enzyme-like activity of nanomaterials is closely related to the catalytically active sites on the surface of nanomaterials [10], thus the catalytic activity in unit area (k cat /area) was calculated. It is found that the IrNPs possess larger k cat /area value than AuNPs [19], Au@PdPt NRs [20] and Fe3O4 nanoparticles [21], suggesting higher catalytic activity of the IrNPs. The catalytic activity is also related to the crystal structure of the IrNPs [17]. According to the XRD and selected area electron diffraction pattern, the IrNPs enclose with {200}, {220} and {311} high-index facets, which possess much higher catalytic activity than basal facets.
The oxidase action of nanomaterials is usually achieved by transformation of DO to various forms of reactive oxygen species (ROS). Hence DPBF, benzoquinone and Vc were used to identify the ROS category produced in the catalytic reaction. DPBF, as one O2ˉ∙ and 1O2 trapper, can not react with other ROS [23]. In the presence of the IrNPs, the fluorescence of the DPBF is quenched and the quenching process is inhibited by the addition of Vc (ROS scavenger), indicating O2ˉ∙ or 1O2 form under the catalytic action of the IrNPs (Fig. S6A). Benzoquinone, a known O2ˉ∙ scavenger, inhibits the catalytic oxidation of TMB, suggesting O2ˉ∙ plays an important role in the oxidase-like activity of the IrNPs (Fig. S6B) [24]. The surface energy of noble metal nanocrystals generally follows the order of γ{111}<γ{100}<γ{110}<γ{hkl}, hence the DO and TMB molecules are more likely absorbed by the (311) facet to decrease the surface energy of the IrNPs [6]. The absorbed TMB molecule donates electron from the amino groups to the IrNPs, resulting in an increase of electron density. The subsequent electron transfer from the IrNPs to DO leads to the formation of O2ˉ∙, which can oxidize TMB to form blue colored oxTMB.
Determination of DO
The TMB can be readily oxidized by DO in the presence of the IrNPs. With the injection of increasing amount of water with previously standardized DO concentration, the color of the formed product intensifies in accordance with the DO concentration. Therefore, a rapid colorimetric method is developed for the determination of DO based on the oxidation of TMB by DO under catalytic action of the IrNPs. As shown in Fig. 3a, the absorbance values increase proportionately with the increase of DO concentration from 12.5 to 257.5 μM. The regression equation is, A = 0.00159 + 0.00066 CDO, with the correlation coefficient (r) of 0.9996 and the limit of detection of 4.7 μM based on the 3σ criterion (Fig. 3b). The color change of TMB from colorless to blue can be readily observed by bare eyes, suggesting that the IrNPs oxidase mimics is applicable for the determination of DO (Fig. 3c).
a UV-vis absorbance spectra of TMB oxidized by different DO concentrations under the catalytic action of the IrNPs; b The linear correlation between the absorbance value at 652 nm and DO concentration (n = 11); c the corresponding photographs under visible light. Reaction conditions: 20 mM NaAc buffer (pH 3.5), 250 μM TMB, 34.46 nM IrNPs, room temperature
To evaluate the selectivity of the method, the effects of commonly encountered ions on the TMB oxidation were investigated. Results show that anions, including Cl−, I−, Br−, F−, SO4 2− and NO3 −, had no effect, while Fe2+, Cu2+, Fe3+ and Hg2+ showed some inhibition effects (Fig. 4). We speculated that Fe2+ consumed the DO in reaction system due to its reducing property. Fortunately the effect from the Fe2+ may not be a serious issue in the real samples, because Fe2+ can be readily oxidized to Fe3+. The inhibition effect of Cu2+, Fe3+ and Hg2+ can be eliminated after addition of EDTA. It is worthy to note, however, that this colorimetric sensor may not be suitable for the determination of DO in colored samples or samples containing peroxides. These species will interfere in the determination of DO, because that colored sample will interfere the light absorption and peroxides with strong electron withdrawing ability can also oxidize the TMB to form oxTMB [11].
To test the applicability of the colorimetric sensor, the concentrations of DO in tap water, bottled water and lake water were determined. The Winkler titration method was used to evaluate the accuracy of IrNPs colorimetric sensor by comparing the analysis results. In Table S1, the average DO concentrations of tap water, bottled water and lake water obtained from the IrNPs sensor are 203.8 μM, 248.7 μM and 101.4 μM, respectively. They agree well with the results of Winkler titration method (204.2 μM, 248.3 μM and 101.5 μM).
Comparison of DO determination methods
The assay shows a number of advantages over other existing methods [12, 25], especially when the overall analysis time, selectivity and simplicity of measurements are considered (Table 2). The traditional Clark sensor, for example, often suffers from serious interferences from chlorine, ozone and nitrogen oxides and it requires routine maintenance to keep long-term stability. Photoluminescence (PL) sensors exhibit the high sensitivity and fast response time [26,27,28,29], and have been successfully applied to DO analysis of complex systems [30, 31]. However, they can be interfered by the strong background emission from biological samples and require sophisticated and expensive equipment. Compared with nanomaterial based fluorometric methods [32, 33], this one is rapid and environmentally friendly. Hence, it is likely to have a large potential for the determination of DO in environment and biological samples.
Conclusions
In conclusion, the IrNPs enclosed with {200}, {220}, {311} high-index facets are shown to display high oxidase-like activity. Mechanism study suggested that the IrNPs can accept electrons from TMB to form an electron rich surface and these electrons then transfer to the DO molecules forming O2ˉ∙. Thus the blue colored oxTMB is produced with the help of IrNPs. Based on the color reaction of TMB, the IrNPs can act as a colorimetric chemosensor for the determination of DO. The determination process completes within 60 s and the color change can be observed by bare eyes, suggesting the advantages of fast-response and good selectivity of this colorimetric method. The oxidase property in this work is a first step for the exploration of enzyme mimics of IrNPs and their analogues, thus can inspire further studies of these nanomaterials in analytical, biological and environmental fields.
References
Liu Y, Wu HH, Chong Y, Wamer WG, Xia QS, Cai LN, Nie ZH, Fu PP, Yin JJ (2015) Platinum nanoparticles: efficient and stable catechol oxidase mimetics. ACS Appl Mater Interfaces 7:19709–19717
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Wang LH, Zeng Y, Shen AG, Zhou XD, Hu JM (2015) Three dimensional nano-assemblies of noble metal nanoparticle–infinite coordination polymers as specific oxidase mimetics for degradation of methylene blue without adding any cosubstrate. Chem Commun 51:2052–2055
Čunderlová V, Hlaváček A, Horňáková V, Peterek M, Němeček D, Hampl A, Eyer L, Skládal P (2016) Catalytic nanocrystalline coordination polymers as an efficient peroxidase mimic for labeling and optical immunoassays. Microchim Acta 183(2):651–658
Nasir M, Nawaz MH, Latif U, Yaqub M, Akhtar H, Rahim A (2017) An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta 184:323–342
Quan ZW, Wang YX, Fang JY (2013) High-index faceted noble metal nanocrystals. Acc Chem Res 46:191–202
Periasamy AP, Roy P, Wu WP, Huang YH, Chang HT (2016) Glucose oxidase and horseradish peroxidase like activities of cuprous oxide/polypyrrole composites. Electrochim Acta 215:253–260
Mévellec V, Roucoux A, Ramirez E, Philippot K, Chaudret B (2004) Surfactant-stabilized aqueous iridium(0) colloidal suspension: an efficient reusable catalyst for hydrogenation of arenes in biphasic media. Adv Synth Catal 346:72–76
Chakrapani K, Sampath S (2014) The morphology dependent electrocatalytic activity of Ir nanostructures towards oxygen reduction. Phys Chem Chem Phys 16:16815–16823
Su H, Liu DD, Zhao M, Hu WL, Xue SS, Cao Q, Le XY, Ji LN, Mao ZW (2015) Dual-enzyme characteristics of polyvinylpyrrolidone-capped iridium nanoparticles and their cellular protective effect against H2O2-induced oxidative damage. ACS Appl Mater Interfaces 7:8233–8242
Cui ML, Zhou JD, Zhao Y, Song QJ (2017) Facile synthesis of iridium nanoparticles with superior peroxidase-likeactivity for colorimetric determination of H2O2 and xanthine. Sensors Actuators B Chem 243:203–210
Martz T, Takeshita Y, Rolph R, Bresnahan P (2012) Tracer monitored titrations: measurement of dissolved oxygen. Anal Chem 84:290–296
Stetter JR, Li J (2008) Amperometric gas sensors-a review. Chem Rev 108:352–366
Cui ML, Zhao Y, Wang C, Song QJ (2016) Synthesis of 2.5 nm colloidal iridium nanoparticles with strong surface enhanced Raman scattering activity. Microchim Acta 183:2047–2053
Purich DL (2010) Enzyme kinetics catalysis and control. Elsevier Inc
Huang XQ, Zhao ZP, Fan JM, Tan YM, Zheng NF (2011) Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J Am Chem Soc 133:4718–4721
Asati A, Santra S, Kaittanis C, Nath S, Perez JM (2009) Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed 48:2308–2321
Dong YM, Zhang JJ, Jiang PP, Wang GL, Wu XM, Zhao H, Zhang C (2015) Superior peroxidase mimetic activity of carbon dots–Pt nanocomposites relies on synergistic effects. New J Chem 39:4141–4146
Luo WJ, Zhu CF, Su S, Li D, He Y, Huang Q, Fan CH (2010) Self-catalyzed, self-limiting growth of glucose oxidase-mimicking gold nanoparticles. ACS Nano 4:7451–7458
Zhang K, Hu XN, Liu JB, Yin JJ, Hou S, Wen T, He WW, Ji YL, Guo YT, Wang Q, Wu XC (2011) Formation of PdPt alloy Nanodots on gold Nanorods: tuning oxidase-like activities via composition. Langmuir 27:2796–2803
Kim MI, Shim JM, Li TH, Lee JW, Park HG (2011) Fabrication of nanoporous nanocomposites entrapping Fe3O4 magnetic nanoparticles and oxidases for colorimetric biosensing. Chem Eur J 17:10700–10707
Shi WB, Wang QL, Long YJ, Cheng ZL, Chen SH, Zheng HZ, Huang YM (2011) Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun 47:6695–6697
Ohyashiki T, Nunomura M, Katoh T (1999) Detection of superoxide anion radical in phospholipid liposomal membrane by fluorescence quenching method using 1,3-diphenylisobenzofuran. Biochim Biophys Acta 1421:131–139
Yang WS, Hao JH, Zhang Z, Zhang BL (2015) PB@Co3O4 nanoparticles as both oxidase and peroxidase mimics and their application for colorimetric detection of glutathione. New J Chem 39:8802–8806
Wu CC, Yasukawa T, Shiku H, Matsue T (2005) Fabrication of miniature Clark oxygen sensor integrated with microstructure. Sensors Actuators B Chem 110:342–349
Nagl S, Baleizão C, Borisov SM, Schäferling M, Berberan-Santos MN, Wolfbeis OS (2007) Optical sensing and imaging of trace oxygen with record response. Angew Chem Int Ed 46:2317–2319
Meier RJ, Schreml S, Wang XD, Landthaler M, Babilas P, Wolfbeis OS (2011) Simultaneous photographing of oxygen and pH in vivo using sensor films. Angew Chem Int Ed 50:10893–10896
Gao Y, Chen T, Yamamoto S, Miyashita T, Mitsuishi M (2015) Superhydrophobic porous surfaces: dissolved oxygen sensing. ACS Appl Mater Interfaces 7:3468–3472
Chu CS, Chuang CY (2015) Optical fiber sensor for dual sensing of dissolved oxygen and Cu2+ ions based on PdTFPP/CdSe embedded in sol–gel matrix. Sensors Actuators B Chem 209:94–99
Yoshihara T, Yamaguchi Y, Hosaka M, Takeuchi T, Tobita S (2012) Ratiometric molecular sensor for monitoring oxygen levels in living cells. Angew Chem Int Ed 124:4224–4227
Nichols AJ, Roussakis E, Klein OJ, Evans CL (2014) Click-assembled, oxygen-sensing nanoconjugates for depth-resolved, near-infrared imaging in a 3D cancer model. Angew Chem Int Ed 53:3671–3674
Chu CS, Lo YL (2011) Highly sensitive and linear calibration optical fiber oxygen sensor based on Pt(II) complex embedded in sol-gel matrix. Sensors Actuators B Chem 155:53–57
Nagl S, Baleizão C, Borisov SM, Schäferling M, Berberan-Santos MN, Wolfbeis OS (2007) Optical sensing and imaging of trace oxygen with record response. Angew Chem Int Ed 46:2317–2319
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51502115, 21403090), and the Enterprise university-research prospective program Jiangsu Province (BY 2015019-22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We have declared that we have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 14.1 MB)
Rights and permissions
About this article
Cite this article
Cui, M., Zhao, Y., Wang, C. et al. The oxidase-like activity of iridium nanoparticles, and their application to colorimetric determination of dissolved oxygen. Microchim Acta 184, 3113–3119 (2017). https://doi.org/10.1007/s00604-017-2326-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2326-9