Abstract
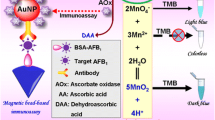
The authors describe a photoelectrochemical (PEC) immunoassay for determination of aflatoxin B1 (AFB1) in foodstuff. The competitive immunoreaction is carried out on a microplate coated with a capture antibody against AFB1 using AFB1-bovine serum albumin (BSA)-liposome-coated mesoporous silica nanoparticles (MSN) loaded with L-cysteine as a support. The photocurrent is produced by a photoactive material consisting of cerium-doped Bi2MoO6. Initially, L-cysteine acting as the electron donor is gated in the pores by interaction between mesoporous silica and liposome. Thereafter, AFB1-BSA conjugates are covalently bound to the liposomes. Upon introduction of the analyte (AFB1), the labeled AFB1-BSA complex competes with the analyte for the antibody deposited on the microplate. Accompanying with the immunocomplex, the liposomes on the MSNs are lysed upon addition of Triton X-100. This results in the opening of the pores and in a release of L-cysteine. Free cysteine then induces the electron-hole scavenger of the photoactive nanosheets to increase the photocurrent. The photocurrent (relative to background signal) increases with increasing AFB1 concentration. Under optimum conditions, the photoactive nanosheets display good photoelectrochemical responses, and allow the detection of AFB1 at a concentration as low as 0.1 pg·mL−1 within a linear response in the 0.3 pg·mL−1 to 10 ng·mL−1 concentration range. Accuracy was evaluated by analyzing naturally contaminated and spiked peanut samples by using a commercial AFB1 ELISA kit as the reference, and well-matching results were obtained.

Schematic presentation of a photoelectrochemical immunoassay for AFB1. It is based on the use of Ce-doped Bi2MoO6 nanosheets and of liposome-coated mesoporous silica nanoparticles loaded with L-cysteine.





Similar content being viewed by others
References
Torchilin V (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–160
Edwards K, Baeumner A (2006) Liposomes in analyses. Talanta 68:1421–1431
Liu Q, Boyd B (2013) Liposomes in biosensors. Analyst 138:391–409
Bui M, Ahmed S, Abbas A (2015) A single-digit pathogen and attomolar detection with the bare eye using liposome-amplified plasmonic immunoassay. Nano Lett 15:6239–6246
Zhuang J, Han B, Liu W, Zhou J, Liu K, Yang D, Tang D (2018) Liposome-amplified photoelectrochemical immunoassay for highly sensitive monitoring of disease biomarkers based on a split-type strategy. Biosens Bioelectron 99:230–236
Liu M, Gan L, Chen L, Zhu D, Zhi Z, Hao X, Chen L (2012) A novel liposome-encapsulated hemoglobin/silica nanoparticle as an oxygen carrier. Int J Pharm 427:354–357
Huang Y, Chung T, Wu C (1998) Effect of saturated/unsaturated phosphatidylcholine ratio on the stability of liposome-encapsulated hemoglobin/Silica. Int J Pharm 172:161–167
Tan Q, Zhang R, Kong R, Kong W, Zhao W, Qu F (2018) Detection of glutathione based on MnO2 nanosheet-gated mesoporous silica nanoparticles and target induced release of glucose measured with a portable glucose meter. Microchim Acta 185:44
Knežević N, Trewyn B, Lin V (2010) Functionalized mesoporous silica nanoparticle-based visible light responsive controlled release delivery system. Chem Commun 47:2817–2819
Martínez-Carmona M, Lozano D, Baeza D, Colilla M, Vallet-Regí M (2017) A novel visible light responsive nanosystem for cancer treatment. Nanoscale 9:15967–15973
Muhammad M, Guo M, Qi W, Sun F, Wang A, Guo Y, Zhu G (2011) pH-triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J Am Chem Soc 13:8778–8781
Park C, Kim H, Kim C (2009) Enzyme responsive nanocontainers with cyclodextrin gatekeepers and synergistic effects in release of guests. J Am Chem Soc 131:16614–16615
Chung P, Kumar R, Pruski M, Lin V (2008) Temperature responsive solution partition of organic–inorganic hybrid poly(N-isopropylacrylamide)-coated mesoporous silica nanospheres. Adv Funct Mater 18:1390–1398
Luo Z, Hu Y, Cai K, Ding X, Zhang Q, Li M, Ma X, Zhang B, Zeng Y, Li P, Li J, Liu J, Zhao Y (2014) Intracellular redox-activated anticancer drug delivery by functionalized hollow mesoporous silica nanoreservoirs with tumor specificity. Biomaterials 35:7951–7962
Liu J, Stace-Naughton L, Jiang X, Brinker C (2009) Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J Am Chem Soc 131:1354–1355
Liu M, Gan L, Chen L, Zhu D, Xu Z, Zhu D, Hao Z, Chen L (2012) Supramolecular core−shell nanosilica@liposome nanocapsules for drug delivery. Langmuir 28:10725–10732
Cauda V, Engelke H, Sauer A, Arcizet D, Brauchle C, Radler J, Bein T (2010) Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Lett 10:2484–2492
Tang Y, Lai W, Zhang J, Tang D (2017) Competitive photometric and visual ELISA for aflatoxin B1 based on the inhibition of the oxidation of ABTS. Microchim Acta 184:2387–2394
Dai Z, Qin F, Zhao H, Ding J, Liu Y, Chen R (2016) Crystal defect engineering of aurivillius Bi2MoO6 by Ce doping for increased reactive species production in photocatalysis. ACS Catal 6:3180–3192
Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Ji Z, Chang C, Nel A (2015) Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 9:3540–3557
Mortera R, Vivero-Escoto J, I. Slowing I, Garrone E, Onid B, Lin V (2009) Cell-induced intracellular controlled release of membrane impermeable cysteine from a mesoporous silica nanoparticle-based drug delivery system. Chem Commun 22:3219–3221
Chen H, Jiang J, Li Y, Deng T, Shen G, Yu R (2007) A novel piezoelectric immunoagglutination assay technique with antibody-modified liposome. Biosens Bioelectron 22:993–999
Lin Y, Zhou Q, Tang D (2017) Dopamine-loaded liposomes for in-situ amplified photoelectrochemical immunoassay of AFB1 to enhance photocurrent of Mn2+-doped Zn3(OH)2V2O7 nanobelts. Anal Chem 89:11803–11810
Dai Z, Qin F, Zhao H, Ding J, Liu Y, Chen R (2016) Crystal defect engineering of aurivillius Bi2MoO6 by Ce doping for increased reactive species production in photocatalysis. ACS Catal 6:3180–3190
Phuruangrat A, Ekthammathat N, Kuntalue B, Dumrongrojthanath P, Thongtem S, Thongtem T (2014) Hydrothermal synthesis, characterization, and optical properties of Ce doped Bi2MoO6 nanoplates. J Nanomater art. ID:934165
Ahmad M, Pan C, Zhu J (2010) Electrochemical determination of L-cysteine by an elbow shaped, Sb-doped ZnO nanowire-modified electrode. J Mater Chem 20:7169–7147
Costa M, Frías I, Andrade C, Oliveira M (2017) Impedimetric immunoassay for aflatoxin B1 using a cysteine modified gold electrode with covalently immobilized carbon nanotubes. Microchim Acta 184:3205–3213
Lu Z, Chen X, Wang Y, Zheng X, Li C (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182:571–578
Nasirian V, Chabok A, Barati A, Rafienia M, Arabi M, Shamsipur M (2017) Ultrasensitive aflatoxin B1 assay based on FRET from aptamer labelled fluorescent polymer dots to silver nanoparticles labeled with complementary DNA. Microchim Acta 184:4655–4662
Di Nardo F, Baggiani C, Giovannoli C, Spano G, Anfossi L (2017) Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim Acta 184:1295–1304
Tan L, He R, Chen K, Peng R, Huang C, Yang R, Tang Y (2016) Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles. Microchim Acta 183:1469–1477
Yao M, Wang L, Fang C (2016) The chemiluminescence immunoassay for aflatoxin B1 based on functionalized magnetic nanoparticles with two strategies of antigen probe immobilization. Luminescence 32:661–665
Zhang Y, Liao Z, Liu Y, Wan Y, Chang J, Wang H (2017) Flow cytometric immunoassay for aflatoxin B1 using magnetic microspheres encoded with upconverting fluorescent nanocrystals. Microchim Acta 184:1471–1479
Tang D, Lin Y, Zhou Q, Lin Y, Li P, Niessner R, Knopp D (2014) Low-cost and highly sensitive immunosensing platform for aflatoxins using one-step competitive displacement reaction mode and portable glucometer-based detection. Anal Chem 86:11451–11458
Nakamichi I, Iwaku M, Fusayama T (1983) Bovine teeth as possible substitutes in the adhesion test. J Dent Res 62:1076–1081
Acknowledgements
Authors thank the National Natural Science Foundation of China (21475025 & 21675029), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT15R11) for financial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1112 kb)
Rights and permissions
About this article
Cite this article
Lin, Y., Zhou, Q., Zeng, Y. et al. Liposome-coated mesoporous silica nanoparticles loaded with L-cysteine for photoelectrochemical immunoassay of aflatoxin B1. Microchim Acta 185, 311 (2018). https://doi.org/10.1007/s00604-018-2848-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2848-9