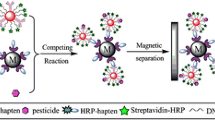
Abstract
A competitive bio-barcode immunoassay is described for the trace detection of parathion in water, pear, cabbage, and rice samples. It is based on amplification by platinum nanoparticle acting as a nanozyme. Gold nanoparticles (AuNPs) were modified with (a) monoclonal antibodies (mAbs) against parathion, and (b) thiolated single-stranded DNA (ssDNA) oligonucleotides. Magnetic nanoparticles (MNPs) were functionalized with ovalbumin coupled with parathion hapten. Parathion and its hapten compete with mAbs on the surface of the AuNPs. Subsequently, the platinum nanoparticles (PtNPs) probe, which was functionalized with the complementary thiolated ssDNA (C-ssDNA), was added to the reaction mixture for the detection of parathion. The signal was catalytically amplified by coupling with platinum nanozyme using teramethylbenzidine and H2O2 as the chromogenic system. The immunoassay has a linear range that extends from 0.01–50 μg·L−1, and the limit of detection is 2.0 × 10−3 μg·L−1. The recoveries and relative standard deviations (RSDs) ranged from 91.1–114.4% and 3.6–15.8%, respectively. The method correlates well with data obtained by gas chromatography-tandem mass spectrometry (GC-MS/MS).

The parathion and the magnetic nanoparticles (MNPs) labelled with hapten-OVA competitively reacted to AuNPs modified with mAbs and thiolated DNA for the detection of parathion. The signal was catalyzed by platinum nanozyme. The limit of detection for parathion is 2.0 ng·L−1.





Similar content being viewed by others
References
Chen G, Jin MJ, Du PF, Zhang C, Cui XY, Zhang YD, She YX, Shao H, Jin F, Wang SS, Zheng LF, Wang J (2017) A sensitive chemiluminescence enzyme immunoassay based on molecularly imprinted polymers solid-phase extraction of parathion. Anal Biochem 530:87–93
Liu J, Parsons L, Pope C (2015) Comparative effects of parathion and chlorpyrifos on endocannabinoid and endocannabinoid-like lipid metabolites in rat striatum. Neurotoxicology 50:20–27
Dagan S (2000) Comparison of gas chromatography–pulsed flame photometric detection–mass spectrometry, automated mass spectral deconvolution and identification system and gas chromatography–tandem. J Chromatogr A 868:229–247
Du PF, Jin MJ, Chen G, Zhang C, Cui XY, Zhang YD, Zhang YX, Zou P, Jiang ZJ, Cao XL, She YX, Jin F, Wang J (2017) Competitive colorimetric triazophos immunoassay employing magnetic microspheres and multi-labeled gold nanoparticles along with enzymatic signal enhancement. Microchim Acta 184:3705–3712
Zhang L, Wang Z, Wen Y, Shi J, Wang J (2015) Simultaneous detection of parathion and imidacloprid using broad-specificity polyclonal antibody in enzyme-linked immunosorbent assay. Anal Methods 7:205–210
Javidi M, Housaindokht MR, Verdian A, Razavizadeh BM (2018) Detection of chloramphenicol using a novel apta-sensing platform based on aptamer terminal-lock in milk samples. Anal Chim Acta 1039:116–123
Kong C, Gao L, Chen Z (2018) Colorimetric adenosine aptasensor based on DNA cycling amplification and salt-induced aggregation of gold nanoparticles. Microchim Acta 185:488
Zou L, Shen R, Ling L, Li G (2018) Sensitive DNA detection by polymerase chain reaction with gold nanoparticles. Anal Chim Acta 1038:105–111
Zhan F, Wang T, Iradukunda L, Zhan J (2018) A gold nanoparticle-based lateral flow biosensor for sensitive visual detection of the potato late blight pathogen, Phytophthora infestans. Anal Chim Acta 1036:153–161
Jiang W, Yin H, Zhou Y, Duan J, Li H, Wang M, Waterhouse GIN, Ai S (2018) A novel electrochemiluminescence biosensor for the detection of 5-methylcytosine, TET 1 protein and β-glucosyltransferase activities based on gold nanoclusters-H2O2 system. Sensors Actuators B Chem 274:144–151
Wei W, Wei M, Yin L, Pu Y, Liu S (2018) Improving the fluorometric determination of the cancer biomarker 8-hydroxy-2′-deoxyguanosine by using a 3D DNA nanomachine. Microchim Acta 185:494
Gou D, Xie G, Li Y, Zhang X, Chen H (2018) Voltammetric immunoassay for mycobacterium tuberculosis secretory protein MPT64 based on a synergistic amplification strategy using rolling circle amplification and a gold electrode modified with graphene oxide, Fe3O4 and Pt nanoparticles. Microchim Acta 185:436
He B, Dong X (2018) Aptamer based voltammetric patulin assay based on the use of ZnO nanorods. Microchim Acta 185:462
Wang YH, Xia H, Huang KJ, Wu X, Ma YY, Deng R, Lu YF, Han ZW (2018) Ultrasensitive determination of thrombin by using an electrode modified with WSe2 and gold nanoparticles, aptamer-thrombin-aptamer sandwiching, redox cycling, and signal enhancement by alkaline phosphatase. Microchim Acta 185:502
Chen M, Zhang L, Yang B, Gao M, Zhang X (2018) Facile synthesis of terminal-alkyne bioorthogonal molecules for live -cell surface-enhanced Raman scattering imaging through au-core and silver/dopamine-shell nanotags. Anal Bioanal Chem 410:2203–2210
Jayakumar K, Camarada MB, Rajesh R, Venkatesan R, Ju H, Dharuman V, Wen Y (2018) Layer-by-layer assembled gold nanoparticles/lower-generation (Gn</=3) polyamidoamine dendrimers-grafted reduced graphene oxide nanohybrids with 3D fractal architecture for fast, ultra-trace, and label-free electrochemical gene nanobiosensors. Biosens Bioelectron 120:55–63
Du PF, Jin MJ, Zhang C, Chen G, Cui XY, Zhang YX, Zhang YD, Zou P, Jiang Z, Cao XL, She YX, Jin F, Wang J (2018) Highly sensitive detection of triazophos pesticide using a novel bio-bar-code amplification competitive immunoassay in a micro well plate-based platform. Sensors Actuators B Chem 256:457–464
Sutan NA, Manolescu DS, Fierascu I, Neblea AM, Sutan C, Ducu C, Soare LC, Negrea D, Avramescu SM, Fierascu RC (2018) Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater Sci Eng C Mater Biol Appl 93:746–758
Nam JM, Thaxton CS, Mirkin CA (2003) Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301:1884e1886
Du PF, Jin MJ, Chen G, Zhang C, Jiang ZJ, Zhang YX, Zou P, She YX, Jin F, Shao H, Wang SS, Zheng LF, Wang J (2016) A competitive bio-barcode amplification immunoassay for small molecules based on nanoparticles. Sci Rep 6:38114
Zhang C, Du P, Jiang ZJ, Jin MJ, Chen G, Cao X, Cui XY, Zhang YD, Li RX, Abd EAM, Wang J (2018) A simple and sensitive competitive bio-barcode immunoassay for triazophos based on multi-modified gold nanoparticles and fluorescent signal amplification. Anal Chim Acta 999:123–131
Xie J, Zhang X, Wang H, Zheng H, Huang Y, Xie J (2012) Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC, trends anal. Chem. 39:114–129
Fu Y, Zhao X, Zhang J, Li W (2014) DNA-based platinum Nanozymes for peroxidase mimetics. J Phys Chem C 118:18116–18125
He W, Liu Y, Yuan J, Yin JJ, Wu X, Hu X, Zhang K, Liu J, Chen C, Ji Y, Guo Y (2011) Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 32:1139–1147
Cui WW, Wang YY, Yang DD, Du JX (2017) Fluorometric determination of ascorbic acid by exploiting its deactivating effect on the oxidase–mimetic properties of cobalt oxyhydroxide nanosheets. Microchim Acta 184:4749–4755
Roushani M, Shahdost-Fard F (2018) A glassy carbon electrode with electrodeposited silver nanoparticles for aptamer based voltammetric determination of trinitrotoluene using riboflavin as a redox probe. Microchim Acta 185:558
Polsky R, Gill R, Kaganovsky L, Willner I (2006) Nucleic acid-functionalized Pt nanoparticles: catalytic labels for the amplified electrochemical detection of biomolecules. Anal Chem 78:2268–2271
Wang Q, Wei H, Zhang Z, Wang E, Dong S (2018) Nanozyme: an emerging alternative to natural enzyme for biosensing and immunoassay. TrAC, trends anal. Chem. 105:218–224
Wang X, Qin L, Zhou M, Lou Z, Wei H (2018) Nanozyme sensor arrays for detecting versatile Analytes from small molecules to proteins and cells. Anal Chem 90:11696–11702
Roushani M, Shahdost-Fard F (2015) An aptasensor for voltammetric and impedimetric determination of cocaine based on a glassy carbon electrode modified with platinum nanoparticles and using rutin as a redox probe. Microchim Acta 183:185–193
Shahdost-fard F, Salimi A, Khezrian S (2014) Highly selective and sensitive adenosine aptasensor based on platinum nanoparticles as catalytical label for amplified detection of biorecognition events through H2O2 reduction. Biosens Bioelectron 53:355–362
Yan X, Shi HY, Wang MH (2012) Development of an enzyme-linked immunosorbent assay for the simultaneous determination of parathion and imidacloprid. Anal Methods 4:4053
Zhang YQ, Xu ZL, Wang F, Cai J, Dong JX, Zhang JR, Si R, Wang CL, Wang Y, Shen YD, Sun Y, Wang H (2018) Isolation of Bactrian camel single domain antibody for parathion and development of one-step dc-FEIA method using VHH-alkaline phosphatase fusion protein. Anal Chem 90:12886–12892
Xu ZL, Wang Q, Lei HT, Eremin SA, Shen YD, Wang H, Beier RC, Yang JY, Maksimova KA, Sun YM (2011) A simple, rapid and high-throughput fluorescence polarization immunoassay for simultaneous detection of organophosphorus pesticides in vegetable and environmental water samples. Anal Chim Acta 708:123–129
Acknowledgements
The authors gratefully thank the projects of National Key Research Program of China (2016YFD0401101); National Natural Science Foundation (31671938); The Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Agricultural Sciences (1610072018002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 291 kb)
Rights and permissions
About this article
Cite this article
Chen, G., Jin, M., Yan, M. et al. Colorimetric bio-barcode immunoassay for parathion based on amplification by using platinum nanoparticles acting as a nanozyme. Microchim Acta 186, 339 (2019). https://doi.org/10.1007/s00604-019-3433-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3433-6