Summary.
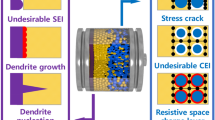
Rechargeable lithium ion cells operate at voltages of 3.5–4.5 V, which is far beyond the thermodynamic stability window of the battery electrolyte. Strong electrolyte reduction and anode corrosion has to be anticipated, leading to irreversible loss of electroactive material and electrolyte and thus strongly deteriorating cell performance. To minimize these reactions, anode and electrolyte components have to be combined that induce the electrolyte reduction products to form an effectively protecting film at the anode/electrolyte interface, which hinders further electrolyte decomposition reactions, but acts as membrane for the lithium cations, i.e. behaving as a solid electrolyte interphase (SEI). This paper focuses on important aspects of the SEI. By using key examples, the effects of film forming electrolyte additives and the change of the active anode material from carbons to lithium storage alloys are highlighted.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received May 30, 2000. Accepted June 14, 2000
Rights and permissions
About this article
Cite this article
Winter, M., Appel, W., Evers, B. et al. Studies on the Anode/Electrolyte Interfacein Lithium Ion Batteries. Monatshefte fuer Chemie 132, 473–486 (2001). https://doi.org/10.1007/s007060170110
Issue Date:
DOI: https://doi.org/10.1007/s007060170110