Abstract
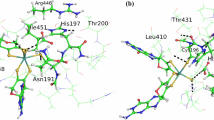
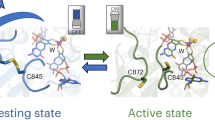
Metal-dependent formate dehydrogenases (Fdh) from prokaryotic organisms are members of the dimethyl sulfoxide reductase family of mononuclear molybdenum-containing and tungsten-containing enzymes. Fdhs catalyze the oxidation of the formate anion to carbon dioxide in a redox reaction that involves the transfer of two electrons from the substrate to the active site. The active site in the oxidized state comprises a hexacoordinated molybdenum or tungsten ion in a distorted trigonal prismatic geometry. Using this structural model, we calculated the catalytic mechanism of Fdh through density functional theory tools. The simulated mechanism was correlated with the experimental kinetic properties of three different Fdhs isolated from three different Desulfovibrio species. Our studies indicate that the C–H bond break is an event involved in the rate-limiting step of the catalytic cycle. The role in catalysis of conserved amino acid residues involved in metal coordination and near the metal active site is discussed on the basis of experimental and theoretical results.












Similar content being viewed by others
Abbreviations
- DFT:
-
Density functional theory
- ES:
-
Enzyme–substrate
- Fdh:
-
Formate dehydrogenase
- PDB:
-
Protein Data Bank
- SeCys:
-
Selenocysteine
- Si :
-
Inorganic sulfur atom
- Tris–HCl:
-
Tris(hydroxymethyl)aminomethane hydrochloride
References
Alberty RA (2001) Arch Biochem Biophys 389:94–109
Heidelberg JF, Seshadri R, Haveman SA, Hemme CL, Paulsen IT, Kolonay JF, Eisen JA, Ward N, Methe B, Brinkac LM, Daugherty SC, Deboy RT, Dodson RJ, Durkin AS, Madupu R, Nelson WC, Sullivan SA, Fouts D, Haft DH, Selengut J, Peterson JD, Davidsen TM, Zafar N, Zhou L, Radune D, Dimitrov G, Hance M, Tran K, Khouri H, Gill J, Utterback TR, Feldblyum TV, Wall JD, Voordouw G, Fraser CM (2004) Nat Biotechnol 22:554–559
Richardson DJ (2000) Microbiology 146(3):551–571
Boyington JC, Gladyshev VN, Khangulov SV, Stadtman TC, Sun PD (1997) Science 275:1305–1308
Khangulov SV, Gladyshev VN, Dismukes GC, Stadtman TC (1998) Biochemistry 37:3518–3528
Jormakka M, Tornroth S, Byrne B, Iwata S (2002) Science 295:1863–1868
Jormakka M, Tornroth S, Abramson J, Byrne B, Iwata S (2002) Acta Crystallogr D Biol Crystallogr 58:160–162
Gladyshev VN, Boyington JC, Khangulov SV, Grahame DA, Stadtman TC, Sun PD (1996) J Biol Chem 271:8095–8100
Moura JJ, Brondino CD, Trincao J, Romao MJ (2004) J Biol Inorg Chem 9:791–799
Brondino CD, Rivas MG, Romao MJ, Moura JJ, Moura I (2006) Acc Chem Res 39:788–796
Raaijmakers H, Teixeira S, Dias JM, Almendra MJ, Brondino CD, Moura I, Moura JJ, Romao MJ (2001) J Biol Inorg Chem 6:398–404
Raaijmakers H, Macieira S, Dias JM, Teixeira S, Bursakov S, Huber R, Moura JJ, Moura I, Romao MJ (2002) Structure 10:1261–1272
Raaijmakers HC, Romao MJ (2006) J Biol Inorg Chem 11:849–854
Costa C, Teixeira M, LeGall J, Moura JJG, Moura I (1997) J Biol Chem 2:198–208
Rivas MG, Gonzalez PJ, Brondino CD, Moura JJ, Moura I (2007) J Inorg Biochem 101:1617–1622
Leopoldini M, Chiodo SG, Toscano M, Russo N (2008) Chemistry 14:8674–8681
Liu MC, Peck HD Jr (1981) J Biol Chem 256:13159–13164
Legall J, Mazza G, Dragoni N (1965) Biochim Biophys Acta 99:385–387
Mota CS, Valette O, Gonzalez PJ, Brondino CD, Moura JJG, Moura I, Dolla A, Rivas MG (2010) J Bacteriol 193:2917–2923
Almendra MJ, Brondino CD, Gavel O, Pereira AS, Tavares P, Bursakov S, Duarte R, Caldeira J, Moura JJ, Moura I (1999) Biochemistry 38:16366–16372
Roux B (1995) Comput Phys Commun 91:275–282
Ensing B, De Vivo M, Liu Z, Moore P, Klein ML (2006) Acc Chem Res 39:73–81
Huber T, Torda AE, van Gunsteren WF (1994) J Comput Aided Mol Des 8:695–708
Laio A, Parrinello M (2002) Proc Natl Acad Sci USA 99:12562–12566
Berg BA, Neuhaus T (1991) Phys Lett B 267:249–253
Ramos MJ, Fernandes PA (2008) Acc Chem Res 41:689–698
Castillo R, Oliva M, Marti S, Moliner V (2008) J Phys Chem B 112:10012–10022
Becke AD (1993) J Chem Phys 98:5648–5652
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200–1211
Cerqueira NM, Fernandes PA, Eriksson LA, Ramos MJ (2006) Biophys J 90:2109–2119
Cerqueira NMFSA, Fernandes PA, Eriksson LA, Ramos MJ (2004) J Mol Struct Theochem 709:53–65
Himo F (2006) Theor Chem Acc 116:232–240
Cerqueira NMFSA, Fernandes PA, Ramos MJ (2011) J Chem Theory Comput 7:1356–1368
Axley MJ, Grahame DA (1991) J Biol Chem 266:13731–13736
Cerqueira NM, Gonzalez PJ, Brondino CD, Romao MJ, Romao CC, Moura I, Moura JJ (2009) J Comput Chem 30:2466–2484
Najmudin S, Gonzalez PJ, Trincao J, Coelho C, Mukhopadhyay A, Cerqueira NM, Romao CC, Moura I, Moura JJ, Brondino CD, Romao MJ (2008) J Biol Inorg Chem 13:737–753
Axley MJ, Bock A, Stadtman TC (1991) Proc Natl Acad Sci USA 88:8450–8454
Castillo R, Oliva M, Marti S, Moliner V (2008) J Phys Chem B 112:10012–10022
Acknowledgments
C.S.M. thanks Fundação para a Ciência e a Tecnologia for funding (grant SFRH/BD/32478/2006). P.J.G. and N.M.F.S.A.C. thank Programa Ciência 2007 and 2008 of Fundação para a Ciência e a Tecnologia. This work was supported by projects PDCT/QUI/57701/2004 and PTDC/QUI/67052/2006 in Portugal and CAID-UNL, CONICET, and SEPCYT in Argentina. C.D.B. thanks to CONICET (Argentina).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mota, C.S., Rivas, M.G., Brondino, C.D. et al. The mechanism of formate oxidation by metal-dependent formate dehydrogenases. J Biol Inorg Chem 16, 1255–1268 (2011). https://doi.org/10.1007/s00775-011-0813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-011-0813-8