Abstract
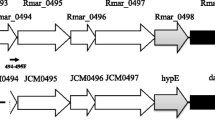
Dye-linked l-proline dehydrogenase catalyzes the oxidation of l-proline in the presence of artificial electron acceptors such as 2, 6-dichloroindophenol and ferricyanide. The enzyme from the hyperthermophilic archaeon Thermococcus profundus was purified and characterized for the first time in archaea by Sakuraba et al. in 2001. In this study, cloning and sequencing analyses of the gene encoding the enzyme and functional analysis of the subunits were performed. The gene formed an operon that consisted of four genes, pdhA, pdhB, pdhF, and pdhX, which are tandemly arranged in the order of pdhA-F-X-B. SDS-PAGE analysis of the purified recombinant enzyme showed four different bands corresponding to α (54 kDa), β (43 kDa), γ (19 kDa), and δ (8 kDa) subunits encoded by pdhA, pdhB, pdhF, and pdhX, respectively, and the molecular ratio of these subunits was determined to be equal. This indicates that the enzyme consists of a heterotetrameric αβγδ structure. Functional analysis of each subunit revealed that the β subunit catalyzed the dye-linked l-proline dehydrogenase reaction by itself and that, unexpectedly, the α subunit exhibited dye-linked NADH dehydrogenase activity. This is the first example showing the existence of a bifunctional dye-linked l-proline/NADH dehydrogenase complex. On the basis of genome analysis, similar gene clusters were observed in the genomes of Pyrococcus horikoshii, Pyrococcus abyssi, Pyrococcus furiosus, and Archaeoglobus fulgidus. These results indicate that the dye-linked l-proline dehydrogenase is a novel type of heterotetrameric amino acid dehydrogenase that might be widely distributed in the hyperthermophilic archaeal strain.










Similar content being viewed by others
References
Allen SW, Senti-Willis A, Maloy SR (1993) DNA sequence of the putA gene from Salmonella typhimurium: a bifunctional membrane-associated dehydrogenase that binds DNA. Nucleic Acids Res 21:1676
Bentrop D, Bertini I, Luchinat C, Nitschke W, Mühlenhoff U (1997) Characterization of the unbound 2[Fe4-S4]-ferredoxin-like photosystem I subunit PsaC from the cyanobacterim Synechococcus elongatus. Biochemistry 36:13692–13637
Brill WJ, Westphal J, Stieghorst M, Davis LC, Shah VK (1974) Detection of nitrogenase components and other nonheme iron proteins in polyacrylamide gels. Anal Biochem 60:237–241
Cafaro V, Scognamiglio R, Viggiani A, Izzo V, Passaro I, Notomista E, Piaz FD, Amoresano A, Casbarra A, Pucci P, Di Donato A (2002) Expression and purification of the recombinant subunits of toluene/o-xylene monooxygenase and reconstitution of the active complex. Eur J Biochem 269:5689–5699
Davis BJ (1964) Disc electrophoresis-2. Method and application to human serum proteins. Ann NY Acad Sci 121:404–427
Esaki N, Shimoi H, Nakajima N, Ohshima T, Tanaka H, Soda K (1989) Enzymatic in situ determination of stereospecificity of NAD-dependent dehydrogenase. J Biol Chem 264:9750–9752
Fish WW (1988) Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol 158:357–364
Gassner G, Wang L, Batie C, Ballou OD (1994) Reaction of phthalate dioxygenase reductase with NADH and NAD: Kinetic and spectral characterization of intermediates. Biochemistry 33:12184–12193
Graham SB, Stephenson JT, Wood JM (1984) Proline dehydrogenase from Escherichia coli K12. J Biol Chem 259:2656–2661
Hugh DC, Ian GY (1983) Stereospecificity and requirements for activity of the respiratory NADH dehydrogenase of Escherichia coli. Biochemistry 22:5754–5760
Lämmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680–685
Leong LM, Tan BH, Ho KK (1992) A specific stain for the detection of nonheme iron proteins in polyacrylamide gels. Anal Biochem 207:317–320
Maloy SR (1987) Escherichia coli and Salmonella typhimurium. In: Neidhardt FC, et al (eds) Cellular and molecular biology, vol 1. American Society for Microbiology, Washington, D.C., pp 1513–1519
Menon AL, Hendrix H, Hutchins A, Verhagen MFJM, Adams MWW (1998) The δ-subunit of pyruvate ferredoxin oxidoreductase from Pyrococcus furiosus is a redox-active, iron-sulfur protein: Evidence for an ancestral relationship with 8Fe-type ferredoxins. Biochemistry 37:12838–12846
Menzel R, Roth J (1981a) Purification of the putA gene product. J Biol Chem 256:9755–9761
Menzel R, Roth J (1981b) Enzymatic properties of the purified putA protein from Salmonella typhimurium. J Biol Chem 256:9762–9766
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Sakuraba H, Takamatsu Y, Satomura T, Kawakami R, Ohshima T (2001) Purification, characterization, and application of a novel dye-linked l-proline dehydrogenase from a hyperthermophilic archaeon, Thermococcus profundus. Appl Environ Microbiol 67:1470–1475
Scarpulla RC, Soffer RF (1978) Membrane-bound proline dehydrogenase from Escherichia coli. J Biol Chem 253:5997–6001
Wierenga RK, Terpstra P, Hol WGJ (1986) Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol 187:101–107
Xia M, Zhu Y, Cao X, You L, Chen Z (1995) Cloning, sequencing and analysis of a gene encoding Escherichia coli proline dehydrogenase. FEMS Microbiol Lett 127:235–242
Acknowledgments
This study was supported by the Pioneering Research Project in Biotechnology of the Ministry of Agriculture, Forestry and Fisheries. R.K. was partially supported by the Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Kawakami, R., Sakuraba, H. & Ohshima, T. Gene and primary structures of dye-linked l-proline dehydrogenase from the hyperthermophilic archaeon Thermococcus profundus show the presence of a novel heterotetrameric amino acid dehydrogenase complex. Extremophiles 8, 99–108 (2004). https://doi.org/10.1007/s00792-003-0368-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0368-x