Abstract
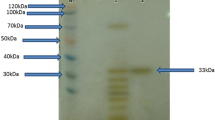
Alkaline protease produced by the halotolerant alkaliphilic Bacillus sp. strain NPST-AK15 was purified to homogeneity by the combination of ammonium sulfate precipitation, anion-exchange and gel permeation chromatography. The purified enzyme was a monomeric protein with an estimated molecular weight of 32 kDa. NPST-AK15 protease was highly active and stable over a wide pH range, with a maximal activity at pH 10.5. The enzyme showed optimum activity at 60 °C and was stable at 30–50 °C for at least 1 h. Thermal stability of the purified protease was substantially improved by CaCl2 (1.1- to 6.6-fold). The K m, V max and k cat values for the enzyme were 2.5 mg ml−1, 42.5 µM min−1 mg−1, and 392.46 × 103 min−1, respectively. NPST-AK15 protease activity was strongly inhibited by PMSF, suggesting that the enzyme is a serine protease. The enzyme was highly stable in NaCl up to 20 % (w/v). Moreover, the purified enzyme was stable in several organic solvents such as diethyl ether, benzene, toluene, and chloroform. In addition, it showed high stability and compatibility with a wide range of surfactants and commercial detergents and was slightly activated by hydrogen peroxide. These features of NPST-AK15 protease make this enzyme a promising candidate for application in the laundry and pharmaceutical industries.







Similar content being viewed by others
References
Abidi F, Chobert J, Haertlé T, Marzouki MN (2011) Purification and biochemical characterization of stable alkaline protease Prot-2 from Botrytis cinerea. Process Biochem 46:2301–2310. doi:10.1016/j.procbio.2011.09.010
Annamalaia N, Rajeswari MV, Sahu SK, Thangavel B (2014a) Purification and characterization of solvent stable, alkaline protease from Bacillus firmus CAS 7 by microbial conversion of marine wastes and molecular mechanism underlying solvent stability. Process Biochem 49:1012–1019. doi:10.1016/j.procbio.2014.03.007
Annamalaia N, Mayavan Veeramuthu Rajeswari MV, Balasubramanian T (2014b) Extraction, purification and application of thermostable and halostable alkaline protease from Bacillus alveayuensis CAS 5using marine wastes. Food Bioprod Process 92:335–342. doi:10.1016/j.fbp.2013.08.009
Beg QK, Gupta R (2003) Purification and characterization of an oxidation-stable, thiol dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb Tech 32:294–304. doi:10.1016/S0141-0229(02)00293-4
Bluum H, Beier Hand Gross HJ (1987) Improved silver staining method of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99. doi:10.1002/elps.1150080203
Deng A, Wu J, Zhang Y, Zhang G, Wen T (2010) Purification and characterization of a surfactant stable high alkaline protease from Bacillus sp. B001. Bioresour Technol 101:7100–7106. doi:10.1016/j.biortech.2010.03.130
Doddapaneni KK, Tatineni R, Vellanki RN, Rachcha S, Anabrolu N, Narakuti V, Mangamoori N (2008) Purification and characterization of a solvent and detergent-stable novel protease from Bacillus cereus. Microbiol Res 164:383–390. doi:10.1016/j.micres.2007.04.005
Dodia MS, Bhimani HG, Rawal CM, Joshi RH, Singh SP (2008) Salt dependent resistance against chemical denaturation of alkaline protease from a newly isolated haloalkaliphilic Bacillus sp. Bioresour Technol 99:6223–6227. doi:10.1016/j.biortech.2007.12.020
Ertan H, Cassel C, Verma A, Poljak A, Charlton T, Aldrich-Wright J, Omar SM, Siddiqui KS, Ricardo C (2015) A new broad specificity alkaline metalloprotease from a Pseudomonas sp. Isolated from refrigerated milk: role of calcium in improving enzyme productivity. J Mol Catal B-Enzym 113:1–8. doi:10.1016/j.molcatb.2014.12.010
George N, Chauhan PS, Kumar V, Puri N, Gupta N (2014) Approach to ecofriendly leather: characterization and application of an alkaline protease for chemical free dehairing of skins and hides at pilot scale. J Clean Prod 79:249–257. doi:10.1016/j.jclepro.2014.05.046
Gessesse A, Hatti-Kaul R, Gashe BA, Mattiasson B (2003) Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enzyme Microb Tech 32:519–524. doi:10.1016/S01410229(02)00324-1
Gohel SD, Singh SP (2015) Thermodynamics of a Ca2+-dependent highly thermostable alkaline protease from a haloalkliphilic actinomycete. Intern J Biol Macromol 72:421–429. doi:10.1016/j.ijbiomac.2014.08.008
Gupta A, Roy I, Patel RK, Singh SP, Khare SK, Gupta MN (2005) One-step purification and characterization of an alkaline protease from haloalkaliphilic Bacillus sp. J Chromatog 20:103–108. doi:10.1016/j.chroma.2005.03.127
Haddar A, Agrebi R, Bougatef A, Hmidet N, Sellami-Kamoun A, Nasri M (2009a) Two detergent stable alkaline serine-proteases from Bacillus mojavensis A21: purification, characterization and potential application as a laundry detergent Additive. Bioresour Technol 100:3366–3373. doi:10.1016/j.biortech.2009.01.061
Haddar A, Bougatef A, Agrebi R, Sellami-Kamoun A, Nasri M (2009b) A novel surfactant-stable alkaline serine-protease from a newly isolated Bacillus mojavensis A21 Purification and characterization. Process Biochem 44:29–35. doi:10.1016/j.procbio.2008.09.003
Horikoshi K (2008) Past, present and future of extremophiles. Extremophiles 12:1–2. doi:10.1007/s00792-007-0127-5
Ibrahim AS, Al-Salamah AA, Elbadawi YB, El-Tayeb MA, Ibrahim SS (2015) Production of extracellular alkaline protease by new halotolerant alkaliphilic Bacillus sp. NPST-AK15 isolated from hyper saline soda lakes (accepted). Electron J Biotechn. doi:10.1016/j.ejbt.2015.04.001
Jain D, Pancha I, Mishra SK, Shrivastav A, Mishra S (2012) Purification and characterization of haloalkaline thermoactive, solvent stable and SDS-induced protease from Bacillus sp.: a potential additive for laundry detergents. Bioresour Technol 115:228–236. doi:10.1016/j.biortech.2011.10.081
Jayakumar R, Jayashree S, Annapurna B, Seshadri S (2012) Characterization of thermostable serine alkaline protease from an alkaliphilic strain Bacillus pumilus MCAS8 and its applications. Appl Biochem Biotechnol 168:1849–1866. doi:10.1007/s12010-012-9902-6
Jellouli K, Ghorbel-Bellaaj O, Ayed H, Manni L, Agrebi R, Nasri M (2011) Alkaline-protease from Bacillus licheniformis MP1: purification, characterization and potential application as a detergents additive and for shrimp waste deproteinization. Process Biochem 46:1248–1256. doi:10.1016/j.procbio.2011.02.012
Joshi S, Satyanarayana T (2013) Characteristics and applications of a recombinant alkaline serine protease from a novel bacterium Bacillus lehensis. Bioresour Technol 131:76–85. doi:10.1016/j.biortech.2012.12.124
Kembhavi AA, Kulkarni A, Pant A (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No. 64. Appl Biochem Biotechnol 38:83–92. doi:10.1007/BF02916414
Kumar RS, Ananthan G, Prabhu AS (2014) Optimization of medium composition for alkaline protease production by Marinobacter sp. GACAS9 using response surface methodology—a statistical approach. Biocatal Agricul Biotech 3:191–197. doi:10.1016/j.bcab.2013.11.005
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Lee S, Jang DJ (2001) Progressive rearrangement of subtilisin Carlsberg into orderly and inflexible conformation with Ca2+ binding. Biophys J 81:2972–2978. doi:10.1016/S0006-3495(01)75937-1
Lowry OH, Rosebrough NJ, Farr AL, Randall J (1951) Protein measurement with the Folin phenol reagent. J Biological Chem 193:265–275
Maruthiah T, Esakkiraj P, Prabakaran G, Palavesam A, Immanuel G (2013) Purification and characterization of moderately halophilic alkaline serine protease from marine Bacillus subtilis AP MSU6. Biocatal Agr Biotech 2:116–119. doi:10.1016/j.bcab.2013.03.001
Mathews CK, Van Holde KE (1990) Dynamic of life: catalysis and control of biochemical reactions. The Benjamin/Cummings publishing company, Inc, USA
Park JY, Park JE, Park JW, Yoon SM, Lee JS (2012) Purification and characterization of a novel alkaline serine protease secreted by Vibrio metschnikovii. Int J Mol Med 29:263–268. doi:10.3892/ijmm.2011.813
Raval VH, Pillai S, Rawa CM, Singh SP (2014) Biochemical and structural characterization of a detergent-stable serine alkaline protease from seawater haloalkaliphilic bacteria. Process Biochem 49:955–962. doi:10.1016/j.procbio.2014.03.014
Sana B, Ghosh D, Saha M, Mukherjee J (2006) Purification and characterization of a salt, solvent, detergent and bleach tolerant protease from a new gamma-Proteobacterium isolated from the marine environment of Sunderbans. Process Biochem 41:208–215. doi:10.1016/j.procbio.2005.09.010
Shah K, Mody K, Keshri J, Jha B (2010) Purification and characterization of a solvent, detergent and oxidizing agent tolerant protease from Bacillus cereus isolated from the Gulf of Khambhat Kunal. J Molecul Catal B: Enzym 67:85–91. doi:10.1016/j.molcatb.2010.07.010
Shankar S, Rao M, Laxman LS (2011) Purification and characterization of an alkaline protease by a new strain of Beauveria sp. Process Biochem 46:579–585. doi:10.1016/j.procbio.2010.10.013
Waghmare SR, Gurav AA, Mali SA, Nadaf NH, Jadhav DB, Sonawane KD (2015) Purification and characterization of novel organic solvent tolerant98 kDa alkaline protease from isolated Stenotrophomonas maltophilia strain SK. Protein Express Purific 107:1–6. doi:10.1016/j.pep.2014.11.002
Wang SL, Yang CH, Liang TW, Yen YH (2008) Optimization of conditions for protease production by Chryseobacterium taeanense TKU001. Bioresour Technol 99:3700–3707. doi:10.1016/j.biortech.2007.07.036
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-BIO2899-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. da Costa.
Rights and permissions
About this article
Cite this article
Ibrahim, A.S.S., Al-Salamah, A.A., El-Badawi, Y.B. et al. Detergent-, solvent- and salt-compatible thermoactive alkaline serine protease from halotolerant alkaliphilic Bacillus sp. NPST-AK15: purification and characterization. Extremophiles 19, 961–971 (2015). https://doi.org/10.1007/s00792-015-0771-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0771-0