Abstract
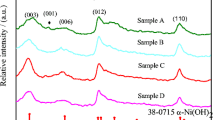
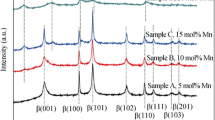
Zn-substituted Ni(OH)2 for alkaline rechargeable batteries was prepared by a chemical coprecipitation method. The structures were characterized by X-ray diffraction and scanning electron microscopy, and the electrochemical performance, including charge–discharge behavior, the proton diffusion coefficient (DH+), and the cycle life, was investigated in detail. The results showed that the charge–discharge potentials of Zn-substituted β-Ni(OH)2 are much higher than those of Zn-substituted α-Ni(OH)2. For a single α (for 30.5–48.4% Zn content) or a single β (from 0 to 9.3% Zn content) phase in the sample, the discharge potentials increase with the increase of Zn content. However, when there is an α and β phase mixture in the sample, the discharge potential decreases with an increase of Zn content. The DH+ values of Zn 0% and Zn 38.1% samples measured by the current-pulse relaxation method are much lower than those of Zn 9.3% and Zn 19.6% samples. DH+ of all the samples decreases with an increase of the depth of discharge. The effects of different Zn contents on the charge–discharge potentials of the nickel electrodes can be attributed to the differences of the electrochemical and diffusion polarization.







Similar content being viewed by others
References
Bode H, Dehmelt K, Witte J (1966) Electrochim Acta 11:1079
Corringan D, Knight SL (1989) J Electrochem Soc 136:613
Barnard R, Randell CF, Tye FF (1980) J Appl Electrochem 10:109
Faure C, Delmas C, Willmann P (1991) J Power Sources 36:497
Kamath PV, Dixit M, Indira L (1994) J Electrochem Soc 141:2956
Sugimoto A, Ishida S, Hanawa K (1999) J Electrochem Soc 146:1251
Liu B, Wang XY, Yuan HT, Zhang YS, Song DY, Zhou ZX (1999) J Appl Electrochem 29:855
Leng YJ, Liu B, Wang FJ, Zhou JX, Xiao Y, Ma ZF (2000) Chin J Power Sources 24:326
Wang CY, Zhong S, Konstantinov K, Walter G, Liu HK (2002) Solid State Ionics 148:503
Hu WK, Noreus D (2003) Chem Mater 15:974
Guerlou-Demourgues L, Delmas C (1993) J Power Sources 45:281
Guerlou-Demourgues L, Delmas C (1996) J Electrochem Soc 143: 561
Tessier C, Guerlou-Demourgues L, Faure C, Basterreix M, Nabias G, Delmas C (2000) J Mater Chem 10:1185
Tessier C, Guerlou-Demourgues L, Faure C, Basterreix M, Nabias G, Delmas C (2000) Solid State Ionics 133:11
Kumagai N, Tanifuji S, Fujiwara T, Tanno K (1992) Electrochim Acta 37:1039
Watanabe K, Kikuoka T (1995) J Appl Electrochem 25:219
Watanabe K, Koseki M, Kumagai N (1996) J Power Sources 58:23
Dean JA (1999) Lange’s handbook of chemistry, 15th edn. McGraw-Hill, New York, p 4.29
Chen H, Wang JM, Pan T, Zhao YL, Zhang JQ, Cao CN (2003) J Electrochem Soc 11:A1399
Acknowledgements
This work was supported by National Natural Science Foundation of China (approval no. 59902004). The authors also gratefully acknowledge the financial support of the Chinese State Key Laboratory for Corrosion and Protection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Wang, J.M., Zhao, Y.L. et al. Electrochemical performance of Zn-substituted Ni(OH)2 for alkaline rechargeable batteries. J Solid State Electrochem 9, 421–428 (2005). https://doi.org/10.1007/s10008-004-0578-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-004-0578-x