Abstract
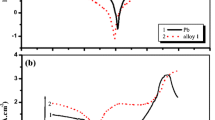
Tetrabutyl ammonium hydrogen sulfate is an ion-paring reagent that has similar properties with ionic liquid. Ionic liquids belong to new branch of salts with unique properties that have ever increasing applications in electrochemical systems especially lithium-ion batteries. For the first time, the effects of tetrabutylammonium hydrogen sulfate (TBAHS) as an electrolyte additive in battery’s electrolyte was studied on the hydrogen and oxygen evolution overpotential and anodic layer formation on lead–antimony–tin grid alloy of lead acid battery by using cyclic voltammetry and linear sweep voltammetry in aqueous sulfuric acid solution. The grid surface morphology after cyclic redox reaction was studied by using scanning electron microscopy. The results show that, by increasing TBAHS concentration in the electrolyte, hydrogen and oxygen overpotential were increased, and so the crystalline structure of PbSO4 layer changed. Also, cyclic voltammogram on carbon–PbO paste electrode shows that with presence of TBAHS in the electrolyte, oxidation and reduction peak current intensively increased and peak potential for oxidation and reduction of PbO were dependent on TBAHS concentration.










Similar content being viewed by others
References
Linden D, Reddy TB (2002) Handbook of batteries, 3rd edn, vol. 23, p 1
Crompton TR (2000) Battery reference book, 3rd edn, vol. 18, p. 5
Francia C, Maja M, Spinelli P (2001) J Power Sources 95:119
Bui N, Mattesco P, Simon P, Steinmetz J, Rocca E (1997) J Power Sources 67:61
Hibbins SG, Timpano FA, Zuliani DJ (1996) US Patent 5,547,634
Rezaei B, Damiri S (2005) J Solid State Electrochem 9:590
Zhong S, Liu HK, Dou SX, Skyllas-Kazacos M (1996) J Power sources 59:123
Pavlov D (1993) J Power Sources 42:345
Garche J, Doring H, Wiesener K (1991) J Power Sources 33:213
Badawy WA, El-Egamy SS (1995) J Power Sources 55:11
Voss E, Hullmeine U, Winsel A (1990) J Power Sources 30:33
Ferreira AL (2001) J Power Sources 94:255
Weighall MJ (2003) J Power Sources 116:219
Ghasemi Z, Tizpar A (2006) Appl Surf Sci 252:3667
Dietz H, Hoogestraat G, Laibach S, von Borstel D, Wiesener K (1995) J Power Sources 53:359
Ohno H (2005) Electrochemical aspects of ionic liquids. Wiley, New York
Sakaebe H, Matsumoto H, Tatsumi K (2007) Electrochim Acta 53:1048
Ishikawa M, Sugimoto T, Kikuta M, Ishiko E, Kono M (2006) J Power Sources 162:658
Markevich E, Baranchugov V, Aurbach D (2006) Electrochem Commun 8:1331
Balducci A, Bardi U, Caporali S, Mastragostino M, Soavi F (2004) Electrochem Commun 6:566
Dai Q, Menzies DB, MacFarlane DR, Batten SR, Forsyth S, Spiccia L, Cheng YB, Forsyth M (2006) C R Chimie 9:617
De Souza RF, Padilha JC, Gonc_alves RS, Dupont J (2003) Electrochem Commun 5:728
Upreti VV, Khurana M, Cox DS, Eddington ND (2006) J Chromatography B 831:156
Vanerkova D, Jandera P, Hrabica J (2007) J Chromatography A 1143:112
Yang X, Hu Z, Yung Chan S, Cher Goh B, Duan W, Chan E, Zhou S (2005) J Chromatography B 821:221
Liu B, Hu XL, Liu J, Zhao YD, Huang ZL (2007) Tetrahedron Lett 48:5958
Tewari N, Dwivedi N, Tripathi RP (2004) Tetrahedron Lett 45:9011
Hirasawa T, Sasaki K, Taguchi M, Kanecho H (2000) J Power Sources 85:44
Babic R, Melikos-Hukoric M, Lajqy N, Brinic S (1994) J Power Sources 52:17
Rusin AI (1987) Modern technology of lead-acid batteries. Energiya, Leningrad, p 182
Culpin B, Rand DAJ (1991) J Power Sources 36:415
Pavlov D (1968) Electrochim Acta 13:2051
Pavlov D, Popova R (1970) Electrochim Acta 15:1483
Ruetschi P (1973) J Electrochem Soc 120:331
Sharpe TF (1977) J Electrochem Soc 124:168
Hampson NA, Kelly S, Peters K (1980) J Appl Electrochem 10:91
Ijomah MN (1987) J Electrochem Soc 134:1960
Webster S, Mitchell PJ, Hampson NA, Dyson DI (1986) J Electrochem Soc 133:137
Pavlov D, Monahov B (1991) J Electroanal Chem 305:57
Pavlov D, Monahov B (1987) J Electroanal Chem 218:135
Gaad Allah AG, El-Rahman HAA, Salih SA, El-Galil MA (1992) J Appl Electrochem 22:571
Guo Y, Chen J, Li L (1992) J Electrochem Soc 139:L–99
Brennan MPJ, Stirrup BN, Hampson NA (1974) J Appl Electrochem 4:497
Acknowledgment
The authors acknowledge the Isfahan University of Technology Council and the Center of Excellency in Sensor and Green Chemistry for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, B., Taki, M. Effects of tetrabutylammonium hydrogen sulfate as an electrolyte additive on the electrochemical behavior of lead acid battery. J Solid State Electrochem 12, 1663–1671 (2008). https://doi.org/10.1007/s10008-008-0547-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0547-x