Abstract
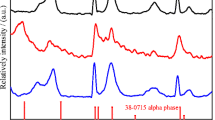
βbc-Nickel hydroxide exhibit non-uniform broadening reflections in their PXRD pattern due to the presence of structural disorder. βbc-Nickel hydroxide electrodes with smaller crystallite size and structural disorder reversibly exchanges 0.9e/Ni. Co/Zn/Ca/Cd-substituted βbc-nickel hydroxide samples also display non-uniform broadening of reflections in their powder X-ray diffraction patterns with smaller crystallite size and exchanges 0.7–0.8e/Ni. Hydrothermal treatment of βbc-nickel hydroxide slurry at 170 °C results in an ordering of reflections in their powder X-ray diffraction pattern with an increased crystallite size. Crystalline β-nickel hydroxide electrode reversibly exchanges 0.3–0.4e/Ni. Hydrothermal-treated Co/Zn/Ca/Cd-substituted βbc-nickel hydroxide slurries at 170 °C display sharp reflections with similar crystallite size and electrochemical activities as that of crystalline β-nickel hydroxide. This clearly demonstrates that partial substitution of Co/Zn/Ca/Cd in the nickel hydroxide matrix does not show any dramatic improvement in their electrochemical activity at 25–30 °C. Structural disordered material with smaller crystallite size delivers electrochemical activity close to theoretical capacity.








Similar content being viewed by others
References
Falk SU, Salkind AJ (1969) Alkaline storage batteries. Wiley, New York
Faure C, Delmas C, Fouassier C (1991) J Power Sources 35:279
Oswald HR, Asper R (1977) In: Lieth RMA (ed) In bivalent metal hydroxides, vol 1. D Reidel Publishing, Holland, p 71
Bode H, Dehmelt K, Witte J (1966) Electrochim Acta 11:1079
Audemer A, Delahaye-Vidal A, Farhi R, Sa-Epee N, Tarascon J-M (1997) J Electrochem Soc 144:2614
Jayashree RS, Kamath PV, Subbanna GN (2000) J Electrochem Soc 147:2029
Hui L, Yunchang D, Jiongliang Y, Zeyun W (1995) J Power Sources 57:137
Ramesh TN, Jayashree RS, Kamath PV (2003) J Electrochem Soc 150:520
Bernard R, Randell C, Tye FL (1981) Power sources, vol. 8. Academic, London, p 401
Ramesh TN, Kamath PV, Shivakumara C (2005) J Electrochem Soc 152:806
Tessier C, Faure C, Guerlou-Demourgues L, Denage C, Nabias G, Delmas C (2002) J Electrochem Soc 149:1136
Constantin DM, Rus EM, Oniciu L, Ghergari L (1988) J Power Sources 74:188
Oshitani M, Sasaki Y, Takashima K (1984) J Power Sources 12:219
Pickett DF, Maloy JT (1978) J Electrochem Soc 125:1026
Pralong V, Chabre Y, Delahaye-Vidal A, Tarascon J-M (2002) Solid State Ionics 147:73
Oshitani M, Yufu H, Takashima K, Tsuji S, Matasumaru Y (1989) J Electrochem Soc 136:1590
Pralog V, Delahaye-Vidal A, Beaudoin B, Gerand B, Leriche JB, Tarascon J-M (2000) J Electrochem Soc 147:1306
Fierro C, Zallen A, Koch J, Fetchenko MA (2006) J Electrochem Soc 153:492
Jun L, Rong L, Hang S (1999) J Power Sources 79:86
Yuan AB, Chang SA, Zhang JQ, Cao CN (1999) J Power Sources 77:178
Yuan AB, Xu NX (2001) J Appl Electrochem 31:45
Oshitani M, Watada M, Shodai K, Kodama M (2001) J Electrochem Soc 148:67
Ramesh TN, Kamath PV, Shivakumara C (2006) Acta Crystallogr B 62:530
Cornilsen BC, Shan X, Loyselle PL (1990) J Power Sources 29:453
Bernard MC, Cortes R, Keddam M, Takenouti H, Bernard P, Senyarich S (1996) J Power Sources 63:247
Delmas C, Tessier C (1997) J Mater Chem 7:1439
Warren BE, Bodenstein P (1966) Acta Crystallogr 20:602
Rajamathi M, Kamath PV, Seshadri R (2000) J Mater Chem 10:503
Radha AV, Kamath PV, Shivakumara C (2007) Acta Crystallogr B 63:243
Bookin AS, Drits VA (1993) Clays Clay Miner 41:551
Faure C, Delmas D, Willmann P (1991) J Power Sources 35:249
Kamath PV, Dixit M, Indira L, Shukla AK, Kumar VG, Munichandraiah N (1994) J Electrochem Soc 141:2956
Audemer A, Delahaye A, Farhi R, Sac-Epée N, Tarascon J-M (1997) J Electrochem Soc 144:2614
Tessier C, Guerlou-Demourgues L, Faure C, Basterreix M, Nabias G, Delmas C (2000) Solid State Ionics 133:11
Casas-Cabanas M, Rodriguez-Carvajal J, Canales-Vazques J, Rose Placin M (2006) J Mater Chem 16:2925
Deabate S, Henn F, Devautour S, Giuntini JC (2003) J Electrochem Soc 150:23
Acknowledgment
T.N.R thanks the Council of Scientific and Industrial Research, GOI, for the award of Senior Research Fellowship (NET) and Research Associate fellowship. P.V.K. thanks the Department of Science and Technology, Government of India (GOI), for financial support. He is a recipient of the Ramanna Fellowship. Authors thank the Solid State and Structural Chemistry Unit of the Indian Institute of Science for powder X-ray diffraction facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh, T.N., Vishnu Kamath, P. The effect of ‘crystallinity’ and structural disorder on the electrochemical performance of substituted nickel hydroxide electrodes. J Solid State Electrochem 13, 763–771 (2009). https://doi.org/10.1007/s10008-008-0591-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0591-6