Abstract
Carbon paste electrode modified with aminated Mobil Catalytic Material Number 41 (MCM-41) was prepared and used for immobilization of K3[Fe(CN)6] in acidic medium, and then electrochemical behavior of modified electrode containing ferricyanide was studied in detail, including pH-dependence and scan rate effect. Cyclic voltammetry studies showed that the electrode reaction is a surface-controlled process at the scan rate range from 5 to 60 mV s−1. Also, the electrocatalytic behavior of modified electrode toward the reduction of H2O2 is reported and the effect of pH on catalytic peak current was discussed. According to experimental results, with increasing solution pH, the catalytic effect of this modified electrode is decreased. Catalytic reduction current of H2O2 increases linearly with its concentration. It has been demonstrated that ferricyanide immobilized on the aminated MCM-41 is a stable catalyst for the electrocatalytic reduction of H2O2.
Similar content being viewed by others
Introduction
Highly ordered three-dimensional mesoporous materials with a pore size from 10 to 100 A° are already technologically important for a variety of applications as catalysts, molecular sieves, and adsorbents. Mesoporous crystalline material Mobil Catalytic Material Number 41 (MCM-41) exhibits extremely high surface area and well-defined pore size, as well as high thermal stability, the synthesis of which was first reported by researchers of Mobil Oil [1, 2]. These materials were prepared by the sol–gel process in the presence of a quaternary ammonium surfactant template and characterized by a cylindrical regular mesopores of monodispersed diameter from 2 to 10 nm [3–6]. Such great porosity and uniform structure provide a very attractive and convenient technique for immobilization of different catalysts.
The internal surfaces of these solids have a large number of silanol groups. Reaction of these silanol groups with various alkoxysilanes has widely been exploited for preparing functionalized MCM-41 by covalent grafting with various organic moieties [7–9]. Functionalized MCM-41 have gained application in the field of electroanalytical chemistry in recent years, such as preconcentration analysis [10, 11], electrocatalysis [12, 13], bioelectrochemistry [14–16], and gas sensors [17–20].
Designing chemically modified electrodes for electrocatalysis [21–24] has been extensively developed because it provides an elegant way to facilitate (accelerate) charge transfer processes. This contributes to decrease the overpotentials often required to perform electrochemical transformations, as well as to increase the intensity of the corresponding voltammetric responses. Until now, electrocatalytic studies with mesoporous silica-based materials are only beginning to be explored [12, 13]. Li et al. [12] have encapsulated 1:12 phosphomolybdic acid in the mesopore channels of MCM-41 materials grafted with amine groups. After incorporation into carbon paste electrodes (CPEs), these materials were found to be efficient catalysts for the reduction of ClO3 – and BrO3 – species, the electrocatalytic properties of 4,4′-bipyridinium units covalently attached to MCM-41 silica walls were also described with respect to the oxidation of 1,4-dihydrobenzoquinone [13].
In the field of electrocatalysis, we have demonstrated the suitability of some modified electrodes for electrocatalytic determination of ascorbic acid [25], hydrazine [26], l-cysteic acid [27], nitrite [28, 29], and carbohydrates [30]. In this work, we used aminated MCM-41 for encapsulation of ferricyanide and used this material for electrocatalytic reduction of H2O2.
Experimental
Apparatus
Electrochemical experiments were performed on 746VA Trace Analyzer Metrohm potantiostat with a Metrohm voltammetry cell in a three electrode configuration. An Ag/AgCl was used as reference electrode and a platinum wire was the auxiliary electrode. Working electrode was carbon paste modified with aminated MCM-41. X-ray powder diffraction data were obtained on a Siemens D5005 diffractometer (German) using Cu Ka radiation.
Chemicals
Pure silica MCM-41 was synthesized by a literature method [31] and was characterized by its X-ray diffraction (XRD) pattern. The grafting reagent 3-aminopropyl triethoxysilane (APTES, Merck) was used without further modification. The electrolyte solutions were HCl and 0.1 M phosphate buffer in pH ranges of 0–1 and 1.5–5, respectively. All other chemicals were of analytical grade and the solutions were prepared with doubly distilled water.
Modification of MCM-41
Amine-functionalized MCM-41 was prepared according to a method in the literature [12]; typically, 0.5 g of MCM-41 was mixed with a chloroform solution of APTES (50 mL, 0.1 M) and stirred at room temperature for 8 h. The modified MCM-41[MCM-41(m)] was filtered and washed with chloroform, and then dried in air. Scheme 1 shows the structure of MCM-41 and MCM-41(m).
Electrode preparation, electrochemical procedures
CPE containing MCM-41(m) was obtained by the homogeneous mixing of graphite particles and MCM-41(m) in a 6:1 mass ratio, and then paraffin oil was added drop wise until a uniformly wetted paste was obtained. A portion of prepared paste was packed into the end of a glass tube with a copper wire as electrical contact. The surface of paste was smoothed on a piece of paper. Then, electrode was immersed in a stirring 1 mM solution of ferricyanide in 1 M HCl for 3 h and washed by stirring in 1 M HCl for 1 h and twice in water. This electrode was studied after reaching a constant current electrochemical behavior. For comparison, similar procedure was performed for bare CPE and carbon paste containing unmodified MCM-41 (MCM-41/CPE).
Results and discussion
Characterization of modified MCM-41
The physico-chemical characteristics of MCM-41 and MCM-41(m) are as expected from previous studies [31]. X-ray diffractogram of MCM-41 (Fig. 1a) shows one main correlation reflection at a 2θ angle of 2.2° and two weaker reflections at higher 2θ angles. The XRD pattern of MCM-41(m) (Fig. 1b) shows a weaker peak at the same 2θ angles, which is due to decreasing channels’ space.
Electrochemical behavior of modified electrode
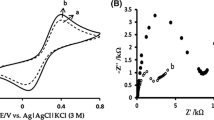
Electrochemical Fig. 2 shows the cyclic voltammograms of ferricyanide accumulated on different electrodes {i.e., CPE, (MCM-41/CPE) and [MCM-41(m)/CPE]} in 1 M HCl before (Fig. 2a) and after (Fig. 2b) washing the electrodes. As can be seen (curve a) at CPE, the ferricyanide does not absorb with respect to MCM-41/CPE or MCM-41(m)/CPE and no redox peak is observed. However, voltammograms b and c exhibit that the absorption occurs at the MCM-41/CPE and MCM-41(m)/CPE, despite the lower internal space of MCM-41(m) with respect to MCM-41; the amount of ferricyanide adsorbed in MCM-41(m)/CPE is more than MCM-41/CPE, which can be attributed to electrostatic interaction between protonated amine and ferricyanide. Also, the residual current in MCM-41(m)/CPE is much lower than that of MCM-41/CPE, which shows improved conductivity of MCM-41(m) via amine groups.
Electrochemical behavior of MCM-41(m)/CPE was studied in pH range 1 to 5. As can be seen in Fig. 3, with an increasing pH, the ΔE p (E pa − E pc) increased, and the anodic and cathodic peak currents decreased; such behavior is different from what is seen for ferricyanide in solution in our previous work [28]. With increasing pH values (replacement of H+ by bulky cations relating to the buffer salts), slower penetration (charge propagation) of bulkier cations to the active centers, as well as decreasing protonation degree of amine groups and, therefore, decreasing electrostatic interaction between amine groups and ferricyanide ions, should, most likely, be the reason for the decrease in rate of charge transfer and peak currents. It must be noted that, when electrode transferred again to pH 1 solution, the low pH voltammogram was restored.
The effect of scan rate on electrochemistry of the modified electrode is shown in Fig. 4. With an increasing scan rate, the anodic peak potential of ferricyanide shifted to a more positive value and the cathodic peak potential shifted in a negative direction (quasireversible behavior). Inset a of Fig. 4 shows the plot of E p vs Log ν for anodic and cathodic peaks. As can be seen at high potential scan rates, the values of E p are proportional to the Log ν with the slopes equal to 2.3RT/αnF and 2.3RT/(1 − α) nF for the cathodic and anodic peaks, respectively, indicated by Laviron [32]. Using the slopes of plots, the value of α was determined as 0.49. Also, peak current value of ferricyanide increased linearly with the increasing scan rate in the range of 5–60 mV s−1 (inset b of Fig. 4), indicating a surface-controlled process.
Main panel: Cyclic voltammograms of MCM-41(m)/CPE in 1 M HCl at different scan rates: 5, 10, 15, 20, 25, 30, 40, 50, 60 mV s−1 from a to i, respectively. a Plot of E p vs log ν for cyclic voltammograms depicted in the main panel. b Relationship between anodic (a) and cathodic (b) peak currents and scan rates
Electrocatalytic reduction of H2O2
In experiments, we found that the carbon paste modified with ferricyanide-MCM-41(m) has a catalytic effect on the reduction of hydrogen peroxide. As is known, the electroreduction of H2O2 requires a large overpotential; no obvious response is observed in the range of +0.7 to −0.3 V on CPE or MCM-41(m)/CPE in 1 M HCl solution containing H2O2 (Fig. 5a and b). However, by use of ferricyanide-MCM-41(m)/CPE, upon addition of H2O2 to the solution, the shape of the cyclic voltammogram of modified electrode changes dramatically with an increase of the reduction current in potential of 0.3 V (compare Fig. 5c and d). This behavior is typical of that expected for mediated reduction as follows:

The effect of pH on catalytic current was investigated. As can be seen in Fig. 6, with increasing pH, the catalytic effect of the modified electrode decreased, which can be attributed to the fact that oxidizing strength of H2O2 depends strongly on pH, and at acidic conditions, hydrogen peroxide acts as a powerful oxidant agent. Also, ferro/ferricyanide will be protonated to some extent, and this may affect the catalytic cycle as well. Therefore, HCl 1 M (pH 0.0) was selected as the optimum medium for determination of H2O2. It must be noted that, in this pH, adding of supporting electrolyte such as KCl and KNO3 has no effect on cyclic voltammograms (not shown) because of the high concentration of protons. Figure 7 shows cyclic voltammetric curves for the H2O2 sensor in 1 M HCl. As can be seen, the reduction current increases linearly with increasing H2O2 in the concentration range of 1 to 30 mM. It is of great importance to mention that the oxidative peak did not disappear after the onset of the reductive catalysis. Such behavior was previously reported for H2O2 reduction on cytochrome c immobilized on the nanometer-scale nickel oxide surfaces on the GC electrode [33]. This phenomenon could be attributed to the fact that most of the adsorbed ferricyanide is probably not catalytically active.
Stability of the modified electrode
After using the modified electrode for electrocatalytic reduction of H2O2, and also after storage for a week of modified electrode at room temperature, it could retain the direct electrochemistry of the immobilized ferrycianide upon the continuous cyclic voltammetric sweep in acidic medium. Storage period of a week in room temperature did not change the currents for the direct electron transfer and the responses to H2O2.
Conclusion
In this work, a modified CPE containing organically modified MCM-41 [MCM-41(m)] was used as a support for immobilization of ferrycianide. The electrochemical behavior of this modified electrode was studied in different pH values and in different potential sweep rates. Results show that the behavior of ferrycianide immobilized in pores of MCM-41(m), especially its pH-dependent electrochemical behavior, is different from the behavior of ferrycianide in aqueous solution. This modified electrode has many advantages, such as fast response, good chemical and mechanical stability, and clean determination of H2O2 without leaching of ferricyanide to the analyte solution. Introducing MCM-41(m) in CPE provides an efficient strategy for the study of electron transfer of ferrycianide and the development of biosensors.
References
Keita B, Nadjo L (1989) Mater Chem Phys 22:77
Keita B, Belhouori A, Nadjo L (1991) J Electroanal Chem 314:345
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710
Moller K, Bein T (1998) Chem Mater 10:2950
Sayari A, Hamoudi S (2001) Chem Mater 13:3151
Stein A, Melde BJ, Schroden RC (2000) Adv Mater 12:1403
Brunel D, Cauvel A, Fajula F, Direnzo F (1995) Stud Surf Sci Catal 97:173
Mereier L, Pinnavia TJ (1997) Adv Mater 9:500
Diaz JF, Balkus KJ, Bedioui F, Kurshev V, Kevan L (1997) Chem Mater 9:61
Walcarius A, Luthi N, Blin JL, Su BL, Lamberts L (1999) Electrochim Acta 44:4601
Walcarius A, Etienne M, Sayen S, Lebeau B (2003) Electroanalysis 15:414
Li L, Li W, Sun C (2002) Electroanalysis 14:368
Domenech A, Alvaro M, Ferrer B, Garcia H (2003) J Phys Chem B 107:12781
Liu B, Hu R, Deng J (1997) Analyst 122:821
Cosnier S, Gondran C, Senillou A, Grätzel M, Vlachopoulos N (1997) Electroanalysis 9:1387
Cosnier S, Senillou A, Grätzel M, Comte P, Vlachopoulos N, Renault NJ, Martelet C (1999) J Electroanal Chem 469:176
Li G, Kawi S (1999) Sens Actuat B 59:1
Yamada T, Zhou HS, Uchida H, Tomita M, Ueno Y, Honma I (2002) Microporous Mesoporous Mater 54:269
Innocenzi P, Martucci A, Guglielmi M, Bearzotti A, Traversa E (2001) Sens Actuat B 76:299
Innocenzi P, Martucci A, Guglielmi M, Bearzotti A, Traversa E, Pivin JC (2001) J Eur Ceram Soc 21:1985
Murray RW (1981) Philos Trans R Soc Lond A 302:253
Zak J, Kuwana T (1983) J Electroanal Chem 150:645
Malinauskas A (1999) Synth Met 107:75
Podlovchenko BI, Andreev VN (2002) Russ Chem Rev 71:837
Ojani R, Raoof JB, Zamani S (2005) Electroanalysis 17:1740
Raoof JB, Ojani R, Ramine M (2007) Electroanalysis 19:597
Raoof JB, Ojani R, Ramine M (2006) Electroanalysis 18:1722
Ojani R, Raoof JB, Zarei E (2006) Electrochim Acta 52:753
Ojani R, Raoof JB, Zarei E (2008) Electroanalysis 20:379
Ojani R, Raoof JB, Afagh PS (2004) J Electroanal Chem 571:1
Zanjanchi MA, Asgari Sh (2004) Solid State Ionics 171:277
Laviron E (1979) J Electroanal Chem 101:19
Bayandori-Moghaddam A, Ganjali MR, Dinarvand R, Razavi T, Saboury AA, Moosavi-Movahedi AA, Norouzi P (2008) J Electroanal Chem 614:83
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ojani, R., Raoof, JB. & Fathi, S. Ferricyanide immobilized within organically modified MCM-41; application for electrocatalytic reduction of hydrogen peroxide. J Solid State Electrochem 13, 837–842 (2009). https://doi.org/10.1007/s10008-008-0615-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0615-2