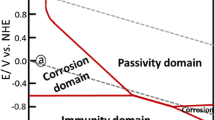
Abstract
The corrosion mechanisms occurring at the homogeneous porous layer was determined assuming that the pores had a cylindrical geometry, and that the initial interface of a carbon steel-CO2 solution behaved as a transmission line (TL). TL modeling quantitatively assessed the impedance distribution and the mesoporous layer formation and evolution at the interface, while describing the physical characteristics of the mesoporous FeCO3 layer at the base and wall within the initial pore. The TL helped to characterize four stages during the interfacial evolution: active, active-porous layer, mixed layer, and the reactive stages. Using TLs helped to quantify the dissolution process and distinguish the mechanisms with good agreement between calculated magnitudes and experimental data.










Similar content being viewed by others
References
Zheng D, Che D, Liu Y (2008) Experimental investigation on gas–liquid two-phase slug flow enhanced carbon dioxide corrosion in vertical upward pipeline. Corros Sci 50:3005–3020
López DA, Pérez T, Simison SN (2003) The influence of microstructure and chemical composition of carbon and low alloy steels in CO2 corrosion. A state-of-the-art appraisal. Mater Des 24:561–575
Foss M, Gulbrandsen E, Sjöblom J (2009) Effect of corrosion inhibitors and oil on carbon dioxide corrosion and wetting of carbon steel with ferrous carbonate deposits. Corrosion 65:3–14
Louafi Y, Ladjouzi MA, Taibi K (2010) Dissolved carbon dioxide effect on the behavior of carbon steel in a simulated solution at different temperatures and immersion times. J Solid State Electrochem 14:1499–1508
Guo XP, Tomoe Y (1999) The effect of corrosion product layer on the anodic and cathodic reactions of carbon steel in CO2 saturated MDEA solutions at 100°C. Corros Sci 41:1391–1402
Zhang GA, Cheng YF (2009) Corrosion of X-65 steel in CO2 saturated oil field formation water in the absence and presence of acetic acid. Corros Sci 51:1589–1595
Nesic S (2007) Key issues related to modeling of internal corrosion of oil and gas pipeline—a review. Corros Sci 49:4308–4338
Nesic S, Nordsveen M, Nyborg R, Stangeland A (2003) A mechanistic model for carbon dioxide corrosion of mild steel in the presence of protective iron carbonate films-part 2: a numerical experiment. Corrosion 59:489–497
Farelas F, Galicia M, Brown B, Nesic S, Castaneda H (2010) Evolution of dissolution processes at the interface of carbon steel corroding in a CO2 environment studied by EIS. Corros Sci 52:509–517
Castaneda H, Benetton XD (2008) SRB-biofilm influence in active corrosion sites formed at the steel-electrolyte interface when exposed to artificial seawater conditions. Corros Sci 50:1169–1183
Castaneda H, Tan B, Saunders J (2010) Electrochemical characterization of the LiCoO2/acetylene carbon ratios for porous electrodes in alkaline lithium aqueous solutions by electrochemical impedance spectroscopy. Electrochim Acta 55:4137–4143
Yu B, Jin Q, Ding D, Li B, Shi AC (2008) Confinement induced morphologies of cylinder-forming asymmetric diblock copolymers. Macromolecules 41:4042–4054
Latz A, Zausch J (2011) Thermodynamic consistent transport theory of Li-ion batteries. J Power Sources 196:3296–3302
Shayegani M, Afshar A, Ghorbani M, Rahmaniyan M (2008) Mild steel carbon dioxide corrosion modeling in aqueous solutions. Corros Eng Sci Technol 43:290–296
Elliott JM, Owen JR (2000) Electrochemical impedance characterization of nanostructured (mesoporous) platinum film. Chem Phys 2:5653–5659
Song H, Jung Y, Lee K, Le Dao H (1999) Electrochemical impedance spectroscopy of porous electrodes: the effect of pore size distribution. Electrochim Acta 44:3513–3519
Castaneda H, Sosa E, Espinosa-Medina MA (2009) Film properties and stability influence on impedance distribution during the dissolution process of low-carbon steel exposed to modified alkaline sour environment. Corros Sci 51:799–806
De Levie R (1963) On porous electrodes in electrolyte solutions. Electrochim Acta 8:751–780
De Levie R (1967) Electrochemical response of porous and rough electrodes. Adv Electroch El Eng 6:329–397
Park JR, Macdonald DD (1983) Impedance studies of the growth of porous magnetite films on carbon steel in high temperature aqueous systems. Corros Sci 23:295–315
De Levie R (1965) The influence of surface roughness of solid electrodes on electrochemical measurements. Electrochim Acta 10:113–130
Pell WG, Conway BE (2001) Voltammetry at a de Levi brush electrode as a model for electrochemical supercapacitor behaviour. J Electroanal Chem 500:121–133
Candy JP, Fouilloux P, Keddam M, Takenouti H (1982) The pore texture of Raney-nickel determined by impedance measurements. Electrochim Acta 27:1585–1593
Meyers JP, Doyle M, Darling RM, Newman J (2000) The impedance response of a porous electrode composed of intercalation particles. J Electrochem Soc 147:2930–2940
Paasch G (2004) Transport in doped conjugated polymers with polarons and bipolarons forming complexes with counter ions. Solid State Ionics 169:87–94
Farelas F (2010) Electrochemical study of the evolution of dissolution and inhibition processes at the interface of 1018 and X65 carbon steels exposed to CO2 environment using a linear flow cell and rotating cylinder electrode. Ph.D. dissertation, Instituto Mexicano del Petroleo
Macdonald DD, Urquidi-Macdonald M (1990) Kramers–Kronig transformation of constant phase impedances. J Electrochem Soc 137:515–518
Sun W, Nesic S (2008) Kinetics of corrosion layer formation: part 1—iron carbonate layers in carbon dioxide corrosion. Corrosion 64:334–346
Franco AA, Schott P, Jallut C, Maschke B (2006) A dynamic mechanistic model of an electrochemical interface. J Electrochem Soc 153:A1053–A1061
Wu S, Orazem ME, Tribollet B, Vivier V (2009) Impedance of a disk electrode with reactions involving an adsorbed intermediate: experimental and simulation analysis. J Electrochem Soc 156:C214–C221
Hinotani S, Ohmori Y, Terasaki F (1985) Effects of Fe3C and Mo2C precipitation on hydrogen diffusivity and hydrogen embrittlement in iron alloys. Mater Sci Eng 76:57–69
Remita E, Tribollet B, Sutter E, Vivier E, Ropital F, Kittel J (2008) Hydrogen evolution in aqueous solution containing dissolved CO2: quantitative contribution of the buffering effect. Corros Sci 50:1433–1440
Li P, Tan TC, Lee JY (1996) Impedance spectra of the anodic dissolution of mild steel in sulfuric acid. Corros Sci 38:1935–1955
Heuer JK, Stubbins JF (1999) An XPS characterization of FeCO3 films from CO2 corrosion. Corros Sci 41:1231–1243
Mora-Mendoza JL, Turgoose S (2002) Fe3C influence on the corrosion rate of mild steel in aqueous CO2 systems under turbulent flow conditions. Corros Sci 44:1223–1246
Castaneda H, Urquidi-Macdonald M (2004) Detecting external failure and corrosion in coated, buried pipelines: transmission line model and experimental verification. Corrosion 60:538–547
Nesic S, Lunde L (1994) Carbon dioxide corrosion of carbon steel in two-phase flow. Corrosion 50:717–727
McDonald DD, Pound BG, Lenhart SJ (1990) The application of electrochemical impedance spectroscopy for characterizing the degradation of Ni(OH)2/NiOOH electrodes. J Power Sources 29:477–502
Nesic S, Sun W (2010) Corrosion in acid gas solutions. In: Richardson TJA (ed) Shreir’s Corrosion, vol 2. Elsevier Science Direct, pp 1270–1298
Rout TK (2007) Electrochemical impedance spectroscopy study on multi-layered coated steel sheets. Corros Sci 49:794–817
Chen HY, Jepson WP (1999) EIS measurement for corrosion monitoring under multiphase flow conditions. Electrochim Acta 44:4453–4464
Li YH, Gregory S (1974) Diffusions of ions in seawater and in deep sea sediments. Geochim Cosmochim Acta 38:703–714
Devos O, Gabrielli C, Tribollet B (2006) Simultaneous EIS and in situ microscope observation on a partially blocked electrode application to scale electrodeposition. Electrochim Acta 51:1413–1422
Videm K, Dugstad A (1989) Corrosion of carbon steel in an aqueous carbon dioxide environment, part 1: solution effects. Mater Perform 28:63–67
Acknowledgments
Monica Galicia acknowledges F. Farelas for the experimental data that was used in this research while he developed part of his Ph.D. at the Institute for Corrosion and Multiphase Technology of Ohio University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castaneda, H., Galicia, M. Proposed model for quantification of dissolution—and evolution of a steel-CO2 solution interface by the transmission line approach. J Solid State Electrochem 16, 3045–3058 (2012). https://doi.org/10.1007/s10008-012-1735-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1735-2