Abstract
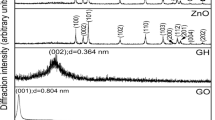
Au/graphene nanocomposites are prepared via a one-pot chemical reduction process at room temperature, using graphene oxide (GO) and chloroauric acid (HAuCl4) as precursors. The obtained Au/graphene nanocomposites are characterized with scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). TEM shows that the Au nanoparticles with size of approximately 8.7 nm disperse randomly on the surface of graphene. XPS confirms that the Au/graphene nanocomposites show a higher atomic percentage of C/O (6.3/1), in contrast to its precursor GO (2.2/1). Electrochemical studies reveal that the Au/graphene nanocomposites have electrochemically active surface area of 9.82 m2 g−1. Besides, the influence of borohydride concentration on the as-prepared Au/graphene nanocomposites is investigated in details by cyclic voltammetry, chronoamperometry, and chronopotentiometry. The results indicate that high concentration of borohydride can significantly improve the electrochemical performance of the Au/graphene catalyst.












Similar content being viewed by others
References
Zhang J, Yang H, Fang J, Zou S (2010) Synthesis and oxygen reduction activity of shape-controlled Pt(3)Ni nanopolyhedra. Nano Lett 10:638–644
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl Catal, B 56:9–35
Duteanu N, Vlachogiannopoulos G, Shivhare MR, Yu EH, Scott K (2007) A parametric study of a platinum ruthenium anode in a direct borohydride fuel cell. J Appl Electrochem 37:1085–1091
Lee H, Park S, Park K, Jung UH, Chun K, Woong C (2008) Development of Au−Pd catalysts supported on carbon for a direct borohydride fuel cell. Res Chem Intermed 34:787–792
Wang GJ, Gao YZ, Wang ZB, Du CY, Wang JJ, Yin GP (2010) Investigation of PtNi/C anode electrocatalysts for direct borohydride fuel cell. J Power Sources 195:185–189
Liu Z, Shamsuzzoha M, Ada ET, Reichert WM, Nikles DE (2007) Synthesis and activation of Pt nanoparticles with controlled size for fuel cell electrocatalysts. J Power Sources 164:472–480
Du HY, Wang CH, Hsu HC, Chang ST, Chen US, Yen SC (2008) Controlled platinum nanoparticles uniformly dispersed on nitrogen-doped carbon nanotubes for methanol oxidation. Diamond Relat Mater 17:535–541
Stakheev AY, Kustov LM (1999) Effects of the support on the morphology and electronic properties of supported metal clusters: modern concepts and progress in 1990s. Appl Catal A 188:3–35
Srinivasan C (2007) Graphene–Mother of all graphitic materials. Curr Sci 92:1338–1339
Muszynski R, Seger B, Kamat PV (2008) Decorating graphene sheets with gold nanoparticles. J Phys Chem C 112:5263–5266
Fang Y, Guo S, Zhu C, Zhai Y, Wang E (2010) Self-Assembly of cationic polyelectrolyte-functionalized graphene nanosheets and gold nanoparticles: A two-Dimensional Heterostructure for Hydrogen Peroxide Sensing. Langmuir 26:11277–11282
Zhang S, Shao Y, Liao HG, Liu J, Aksay IA, Yin G (2011) graphene decorated with PtAu alloy nanoparticles: Facile synthesis and promising application for formic acid oxidation. Chem Mater 23:1079–1081
Guo S, Dong S, Wang E (2009) Three-Dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: Facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation. ACS Nano 4:547–555
Yoo E, Okata T, Akita T, Kohyama M, Nakamura J, Honma I (2009) Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett 9:2255–2259
Xu C, Wang X, Zhu J (2008) Graphene−Metal particle nanocomposites. J Phys Chem C 112:19841–19845
Venkateswara Rao C, Cabrera CR, Ishikawa Y (2011) Graphene−Supported Pt–Au alloy nanoparticles: A highly efficient anode for direct formic acid fuel cells. J Phys Chem C 115:21963–21970
Shang N, Papakonstantinou P, Wang P, Silva SRP (2010) Platinum integrated graphene for methanol fuel cells. J Phys Chem C 114:15837–15841
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Li D, Kaner RB (2008) Graphene–based materials. Science 320:1170–117
Zhou X, Huang X, Qi X, Wu S, Xue C (2009) In situ synthesis of metal nanoparticles on single-layer graphene oxide and reduced graphene oxide surfaces. J Phys Chem C 113:10842–10846
Wang G, Yang J, Park J, Gou X, Wang B (2008) Facile synthesis and characterization of graphene nanosheets. J Phys Chem C 112:8192–8195
Jeong HK, Jin MH, An KH, Lee YH (2009) Structural stability and variable dielectric constant in poly sodium 4-styrensulfonate intercalated graphite oxide. J Phys Chem C 113:13060–13064
Tang H, Ehlert GJ, Lin Y, Sodano HA (2011) Highly efficient synthesis of graphene nanocomposites. Nano Lett 12:84–90
Sharma S, Ganguly A, Papakonstantinou P, Miao X, Li M, Hutchison JL (2010) Rapid microwave synthesis of CO tolerant reduced graphene oxide-supported platinum electrocatalysts for oxidation of methanol. J Phys Chem C 114:19459–19466
Vinodgopal K, Neppolian B, Lightcap IV, Grieser F, Ashokkumar M, Kamat PV (2010) Sonolytic design of graphene−au nanocomposites. Simultaneous and sequential reduction of graphene oxide and Au(III). J Phys Chem Lett 1:1987–1993
Park S, An J, Jung I, Piner RD, An SJ (2009) colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett 9:1593–1597
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D (2008) Chemical oxidation of multiwalled carbon nanotubes. Carbon 46:833–840
Luo DC, Zhang GX, Liu JF, Sun XM (2011) Evaluation criteria for reduced graphene oxide. J Phys Chem C 115:11327–11335
He P, Wang Y, Wang X, Pei F, Wang H, Liu L (2011) Investigation of carbon supported Au–Ni bimetallic nanoparticles as electrocatalyst for direct borohydride fuel cell. Evaluation Criteria for Reduced Graphene Oxide. J Power Sources 196:1042–1047
Maye M, Kariuki N, Luo J, Han L (2004) Electrocatalytic reduction of oxygen: Gold and gold-platinum nanoparticle catalysts prepared by two-phase protocol. Gold Bull 37:217–223
Papadimitriou S, Tegou A, Pavlidou E, Armyanov S (2008) Preparation and characterisation of platinum- and gold-coated copper, iron, cobalt and nickel deposits on glassy carbon substrates. Electrochim Acta 53:6559–6567
Girishkumar G, Rettker M, Underhile R, Binz D (2005) Single-wall carbon nanotube-based proton exchange membrane assembly for hydrogen fuel cells. Langmuir 21:8487–8494
Zhang Z, Chen H, Xing C, Guo M, Xu F (2011) Sodium citrate: A universal reducing agent for reduction / decoration of graphene oxide with au nanoparticles. Nano Res 4:599–611
Előd G (2004) Electrooxidation of borohydride on platinum and gold electrodes: implications for direct borohydride fuel cells. Electrochim Acta 49:965–978
Cheng H, Scott K (2006) Influence of operation conditions on direct borohydride fuel cell performance. J Power Sources
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51072173), Doctoral Fund of Ministry of Education of China (Grant No. 20094301110005), and Key project of Education Department of Hunan Province (Grant No. 11A118).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Wang, X., He, P. et al. Influence of borohydride concentration on the synthesized Au/graphene nanocomposites for direct borohydride fuel cell. J Solid State Electrochem 16, 3929–3937 (2012). https://doi.org/10.1007/s10008-012-1840-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1840-2