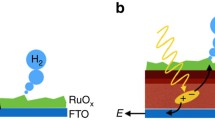
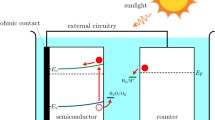
Abstract
Light-driven water-splitting (photoelectrolysis) at semiconductor electrodes continues to excite interest as a potential route to produce hydrogen as a sustainable fuel, but surprisingly little is known about the kinetics and mechanisms of the reactions involved. Here, some basic principles of semiconductor photoelectrochemistry are reviewed with particular emphasis on the effects of slow interfacial electron transfer at n-type semiconductors in the case of light-driven oxygen evolution. A simple kinetic model is outlined that considers the competition between interfacial transfer of photogenerated holes and surface recombination. The model shows that, if interfacial charge transfer is very slow, the build-up of holes at the surface will lead to substantial changes in the potential drop across the Helmholtz layer, leading to non-ideal behavior (Fermi level pinning). The kinetic model is also used to predict the response of photoanodes to chopped illumination and to periodic perturbations of illumination and potential. Recent experimental results obtained for α-Fe2O3 (hematite) photoanodes are reviewed and interpreted within the framework of the model.

















Similar content being viewed by others
References
Fujishima A, Honda K (1972) Nature 238:37–38
Nozik AJ (1977) Bull Am Phys Soc 22:95–95
Grätzel M, Augustyski J (2005) US Patent 6936143
Bolton JR, Strickler SJ, Connolly JS (1985) Nature 316:495–500
Nakamura R, Nakato Y (2004) J Am Chem Soc 126:1290–1298
Nakamura R, Okamura T, Ohashi N, Imanishi A, Nakato Y (2005) J Am Chem Soc 127:12975–12983
Kay A, Cesar I, Grätzel M (2006) J Am Chem Soc 128:15714–15721
Barroso M, Cowan AJ, Pendlebury SR, Grätzel M, Klug DR, Durrant JR (2011) J Am Chem Soc 133:14868–14871
Cowan AJ, Barnett CJ, Pendlebury SR, Barroso M, Sivula K, Grätzel M, Durrant JR, Klug DR (2011) J Am Chem Soc 133:10134–10140
Dotan H, Sivula K, Grätzel M, Rothschild A, Warren SC (2011) Energy and Environ Sci 4:958–964
Sivula K, Le Formal F, Grätzel M (2011) Chem Sus Chem 4:432–449
Wijayantha KGU, Saremi-Yarahmadi S, Peter LM (2011) PCCP 13:5264–5270
Klahr B, Gimenez S, Fabregat-Santiago F, Bisquert J, Hamann TW (2012) Energy and Environ Sci 5:7626–7636
Klahr B, Gimenez S, Fabregat-Santiago F, Hamann T, Bisquert J (2012) J Am Chem Soc 134:4294–4302
Peter LM, Ponomarev EA, Fermin DJ (1997) J Electroanal Chem 427:79–96
Gärtner WW (1959) Phys Rev 116:84
Wilson RH (1977) J Appl Phys 48:4292–4297
Butler MA, Ginley DS (1980) J Mat Sci 15:1–19
Reichman J (1980) Appl Phys Lett 36:574–577
El Guibaly F, Colbow K (1983) J Appl Phys 54:6488–6491
Li J, Peat R, Peter LM (1984) J Electroanal Chem 165:41–59
Peter LM, Wijayantha KGU, Tahir AA (2012) Faraday discuss 155
Cass MJ, Duffy NW, Peter LM, Pennock SR, Ushiroda S, Walker AB (2003) J Phys Chem B 107:5857–5863
Cummings CY, Marken F, Peter LM, Tahir AA, Wijayantha KGU (2012) Chem Commun 48:2027–2029
Cummings CY, Marken F, Peter LM, Wijayantha KGU, Tahir AA (2012) J Am Chem Soc 134:1228–1234
Peter LM, Vanmaekelbergh D (1999) Time and frequency resolved studies of photoelectrochemical kinetics. Adv Electrochem Sci Eng 6:77–163, Weinheim
Peter LM, Li J, Peat R (1984) J Electroanal Chem 165:29–40
Li J, Peter LM (1985) J Electroanal Chem 193:27–47
Peter LM, Li J, Peat R, Lewerenz HJ, Stumper J (1990) Electrochim Acta 35:1657–1664
Ponomarev EA, Peter LM (1995) J Electroanal Chem 397:45–52
Peat R, Peter LM (1987) Ber Bunsen-Ges Phys Chem 91:381–386
Leng WH, Zhang Z, Zhang JQ, Cao CN (2005) J Phys Chem B 109:15008–15023
Schefold J (1992) J Electroanal Chem 341:111–136
Schefold J (1995) J Electroanal Chem 394:35–48
Bertoluzzi L, Bisquert J (2012) J Phys. Chem Lett 3:2517
Acknowledgments
The author thanks Dr. Upul Wijayantha and members of his research group for their collaborative work on hematite electrodes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Alexander Milchev on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
Peter, L.M. Energetics and kinetics of light-driven oxygen evolution at semiconductor electrodes: the example of hematite. J Solid State Electrochem 17, 315–326 (2013). https://doi.org/10.1007/s10008-012-1957-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1957-3