Abstract
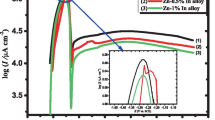
The electrochemical and corrosion behavior of Pb and Pb-In alloys in both phosphoric and sulfuric acid solutions containing various concentrations of phosphoric acid (0.05 to 0.20 M) at different temperatures was studied. Tafel plot and electrochemical impedance spectroscopy (EIS) techniques were used to obtain the experimental data, and the corrosion products formed on the surface were characterized by scanning electron microscopy (SEM). The results of both Tafel plot extrapolation and impedance measurements showed the same trend. Minor indium (0.5 %) alloying with lead significantly reduced corrosion rate in pure phosphoric acid solution. However, opposite behavior arises in the case of alloys containing indium more than 0.5 %, that is, the corrosion is higher than that of lead and alloy I. The corrosion current density decreases in both Pb and alloy I (0.5 %) in 4 M sulfuric acid with increasing the concentration of phosphoric acid up to 0.1 M, and then starts to increase relatively with increasing the additive concentration up to 0.2 M (but still lower than that in pure H2SO4). This exhibits that the higher concentration (0.2 M) of H3PO4 is less inhibitive of alloy I corrosion in H2SO4 solution. However, addition of phosphoric acid in various concentrations to sulfuric acid has little influence to inhibit the corrosion of alloys containing higher indium content (1 to 15 %). SEM photographs showed that the presence of indium as a minor alloying element retards the formation of PbSO4, but the formation of β-PbO2 enhances.














Similar content being viewed by others
References
Garche J, Doring H, Wiesener K (1991) J Power Sources 33:213–220
Visscher W (1976/1977) J Power Sources 1:257-266
Voss E (1988) J Power Sources 24:171–184
Chatelut M, Chah-Bouzziri S, Vittori O, Benayada A (2000) J Solid State Electrochem 4:435–443
Meissner E (1997) J Power Sources 67:135–150
Paleska I, Pruszkowska-Drachal R, Kotowski J, Dziudzi A, Milewski JD, Kopczyk M, Czerwinski A (2003) J Power Sources 113:308–317
Bullock KR, McClelland DH (1977) J Electrochem Soc 124:1478–1482
Bullock KR (1979) J Electrochem Soc 126:360–365
Bullock KR (1979) J Electrochem Soc 126:1848–1853
Doring H, Wiesener K, Garche J, Fischer W (1992) J Power Sources 38:261–272
Venugopalan S (1993) J Power Sources 46:1
Sternberg S, Branzoi V, Apateanu L (1990) J Power Sources 30:177–183
Saminathan K, Jayaprakash N, Rajeswari B, Vasudevan T (2006) J Power Sources 60:1410–1413
El-Sayed A, Shaker AM, Gad El-Kareem H (2003) Bull Chem Soc Jpn 76:1527–1535
Abd El-Rehim SS, Hassan HH, Mohamed NF (2004) Corros Sci 46:1071–1082
Abdel Aal MS, Radwan S, El- Sayed (1983) Br Corros J 18:102–106
Tremont R, De Jasus-Cardona H, Garcia-Orozco J, Castro RJ, Cabcera CR (2000) J Appl Electrochem 30:737–743
Schultze JW, Wippermann K (1987) Electrochim Acta 32:823–831
Clearly HJ, Greene ND (1967) Corros Sci 7:821–831
Li S, Chem HY, Tang MC, Wei WW, Xia ZW, Wu YM, Li WS, Jiang X (2006) J Power Sources 158:914–919
Andersson BO, Ojefors L (1976) J Electrochem Soc 123:824–828
Mohran HS, El-Sayed A, Abd El-lateef HM (2009) J Solid State Electrochem 13:1147–1155
Munoz AG, Saidman SB, Bessone JB (2002) Corros Sci 44:2171–2182
Abdul Azim AA, Sanad SH (1973) Corros Sci 13:861–880
Ŝeruga M, Hasenay D (2001) J Appl Electrochem 31:961–967
El-Sayed A, Shaker AM, Abd El-Lateef HM (2010) Corros Sci 52:72–81
Venugopalan S (1993) J Power Sources 46:1–15
Venugopalan S (1994) J Power Sources 48:371–384
Abd El-Rahman HA, Gad-Allah AG, Salih SA, Abd El-Wahab AM http://www.raco.cat/index.php/afinidad/article/viewFile/273753/361898
Dezhi L, Conway PP, Changqing L (2008) Corros Sci 50:995–1004
Mouanga M, Beroct P (2010) Corros Sci 52:3993–4000
Kalinauskas P, Valsiunas I, Samuleviciene M, Juceliunas E (2001) Corros Sci 43:2083–2092
Sere PR, Culcasi JD, Elsner CI, Sarli ARD (1999) Surf Coat Technol 122:143–149
Barcia OE, Mattos OR, Pebere N, Tribollet B (1993) J Electrochem Soc 140:2825–2832
Deslouis C, Tribollet B, Mengoli G, Musiani M (1988) J Appl Electrochem 18:374–383
Macdonal JR, Impedance spectroscopy: Emphasizing solid materials and systems 1987, by John wiley & Sons. Inc. Chapter 1, page 22
El-Sayed A, Mohran HS, Abd El-Lateef HM (2012) Metall Mater Trans A 43:619–632
El-Sayed A, Mohran HS, Abd El-Lateef HM (2011) J Power Sources 196:6573–6582
El-Sayed A (1997) J Appl Electrochem 27:193–200
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Sayed, AR., Mohran, H.S., Abd El-Lateef, H.M. et al. Effect of indium alloying with lead together with the addition of phosphoric acid in electrolyte to improve lead-acid battery performance. J Solid State Electrochem 19, 1463–1478 (2015). https://doi.org/10.1007/s10008-015-2765-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2765-3