Abstract
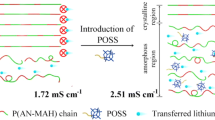
A novel polyhedral oligomeric silsesquioxane (POSS) composite polyacrylonitrile (PAN)-based porous structure gel polymer electrolyte (GPE) is prepared by phase inversion method. The POSS additive filler is firstly obtained in the dehydration condensation reaction of vinyltrimethoxysilane (VTMS) and 3-methacryloxypropyltrimethoxysilane (MPTMS). The composition and structure of synthetic POSS and the prepared POSS composite PAN-based GPEs are investigated. It is found that compared with pure PAN-based GPE, the POSS composite PAN-based GPE with 8 wt.% POSS presents the homogeneous pore distribution and abundant electrolyte uptake (540.4 wt.%), which endows GPE-8% with the excellent comprehensive performances: the highest ionic conductivity of 2.62 × 10−3 S cm−1 at room temperature, the higher lithium ion transference number of 0.38, the good compatibility with lithium anode, and the higher electrochemical stability window of 5.7 V (vs. Li/Li+). At 0.2 C, the GPE-8%-based lithium ion battery produces a satisfactory discharge capacity of 140 mAh g−1.

ᅟ









Similar content being viewed by others
References
Armand M, Tarascon JM (2008) Building better batteries. Nature 451(7179):652–657. https://doi.org/10.1038/451652a
Jeschke S, Mutke M, Jiang ZX, Alt B, Wiemhofer HD (2014) Study of carbamate-modified disiloxane in porous PVDF-HFP membranes: new electrolytes/separators for lithium ion batteries. Chem Phys Chem 15(9):1761–1771. https://doi.org/10.1002/cphc.201400065
Li H, Lin CE, Shi JL, Ma XT, Zhu BK, Zhu LP (2014) Preparation and characterization of safety PVDF/P(MMA-co-PEGMA) active separators by studying the liquid electrolyte distribution in this kind of membrane. Electrochim Acta 115:317–325. https://doi.org/10.1016/j.electacta.2013.10.183
Liao YH, Chen TT, Luo XY, Fu Z, Li XP, Li WS (2016) Cycling performance improvement of polypropylene supported poly (vinylidene fluoride-co-hexafluoropropylene)/maleic anhydride-grated-polyvinylidene fluoride based gel electrolyte by incorporating nano-Al2O3 for full batteries. J Membr Sci 507:126–134. https://doi.org/10.1016/j.memsci.2016.02.001
Wang FX, Xiao SY, Shi Y, Liu LL, Zhu YS, Wu YP, Wang JZ, Holze R (2013) Spinel LiNi(x)Nn(2-x)O(4) as cathode material for aqueous rechargeable lithium batteries. Electrochim Acta 93:301–306. https://doi.org/10.1016/j.electacta.2013.01.106
Zuo X, Liu XM, Cai F, Yang H, Shen XD, Liu G (2012) A novel all-solid electrolyte based on a co-polymer of poly-(methoxy/hexadecal-poly(ethylene glycol) methacrylate) for lithium-ion cell. J Mater Chem 22(41):22265–22271. https://doi.org/10.1039/c2jm34270g
Hassoun J, Panero S, Reale P, Scrosati B (2009) A new, safe, high-rate and high-energy polymer lithium-ion battery. Adv Mater 21(47):4807–4810. https://doi.org/10.1002/adma.200900470
Lu QW, Fang JH, Yang J, Miao RR, Wang JL, Nuli YN (2014) Novel cross-linked copolymer gel electrolyte supported by hydrophilic polytetrafluoroethylene for rechargeable lithium batteries. J Membr Sci 449:176–183
Chen ZH, Ren Y, Jansen AN, Lin CK, Weng W, Amine K (2013) New class of nonaqueous electrolytes for long-life and safe lithium-ion batteries. Nat Commun 4:1513. https://doi.org/10.1038/ncomms2518
Lee YS, Lee JH, Choi JA, Yoon WY, Kim DW (2013) Cycling characteristics of lithium powder polymer batteries assembled with composite gel polymer electrolytes and lithium powder anode. Adv Funct Mater 23(8):1019–1027. https://doi.org/10.1002/adfm.201200692
Yang PX, Liu L, Li LB, Hou J, Xu YP, Ren XF, An MZ, Li N (2014) Gel polymer electrolyte based on polyvinylidenefluoride-co-hexafluoropropylene and ionic liquid for lithium ion battery. Electrochim Acta 115:454–460. https://doi.org/10.1016/j.electacta.2013.10.202
Chen ST, Li X, Yao K, Tay FEH, Kumar A, Zeng KY (2012) Self-polarized ferroelectric PVDF homopolymer ultra-thin films derived from Langmuir-Blodgett deposition. Polymer 53(6):1404–1408. https://doi.org/10.1016/j.polymer.2012.01.058
Chen TT, Liao YH, Wang XS, Luo XY, Li XP, Li WS (2016) Investigation on high-safety lithium ion battery using polyethylene supported poly(methyl methacrylate-acrylonitrile-butyl acrylate) copolymer based gel electrolyte. Electrochim Acta 191:923–932. https://doi.org/10.1016/j.electacta.2016.01.053
Peng XX, Zhou L, Jing B, Cao Q, Wang XY, Tang XL, Zeng J (2016) A high-performance electrospun thermoplastic polyurethane/poly(vinylidene fluoride-co-hexafluoropropylene) gel polymer electrolyte for Li-ion batteries. J Solid State Electrochem 20(1):255–262. https://doi.org/10.1007/s10008-015-3030-5
Rao MM, Liu JS, Li WS, Liang Y, Liao YH, Zhao LZ (2009) Performance improvement of poly(acrylonitrile-vinyl acetate) by activation of poly(methyl methacrylate). J Power Sources 189(1):711–715. https://doi.org/10.1016/j.jpowsour.2008.08.049
Tang CY, Hackenberg K, Fu Q, Ajayan PM, Ardebili H (2012) High ion conducting polymer nanocomposite electrolytes using hybrid nanofillers. Nano Lett 12(3):1152–1156. https://doi.org/10.1021/nl202692y
Yang YQ, Chang Z, Li MX, Wang XW, Wu YP (2015) A sodium ion conducting gel polymer electrolyte. Solid State Ionics 269:1–7. https://doi.org/10.1016/j.ssi.2014.11.015
Zhu YS, Xiao SY, Shi Y, Yang YQ, Hou YY, Wu YP (2014) A composite gel polymer electrolyte with high performance based on poly(vinylidene fluoride) and polyborate for lithium ion batteries. Adv Energy Mater 4:375–379
Lin CW, Hung CL, Venkateswarlu M, Hwang BJ (2005) Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J Power Sources 146(1-2):397–401. https://doi.org/10.1016/j.jpowsour.2005.03.028
Liu Y, Lee JY, Hong L (2004) In situ preparation of poly(ethylene oxide)–SiO2 composite polymer electrolytes. J Power Sources 129(2):303–311. https://doi.org/10.1016/j.jpowsour.2003.11.026
Nara H, Momma T, Osaka T (2008) Feasibility of an interpenetrated polymer network system made of di-block copolymer composed of polyethylene oxide and polystyrene as the gel electrolyte for lithium secondary batteries. Electrochemistry 76(4):276–281. https://doi.org/10.5796/electrochemistry.76.276
Yue Z, McEwen IJ, Cowie JMG (2003) Novel gel polymer electrolytes based on a cellulose ester with PEO side chains. Solid State Ionics 156(1-2):155–162. https://doi.org/10.1016/S0167-2738(02)00595-7
Choi BK, Kim YW, Shin HK (2000) Ionic conduction in PEO–PAN blend polymer electrolytes. Electrochim Acta 45(8-9):1371–1374. https://doi.org/10.1016/S0013-4686(99)00345-X
Huai Y, Deng J, Li R, Deng Z, Suo J (2013) Polymer/colloid dual-phase electrolyte membrane for rechargeable lithium batteries. J Solid State Electrochem 17(1):209–215. https://doi.org/10.1007/s10008-012-1877-2
Lee KH, Lee YG, Park JK, Seung DY (2000) Effect of silica on the electrochemical characteristics of the plasticized polymer electrolytes based on the P(AN-co-MMA) copolymer. Solid State Ionics 133(3-4):257–263. https://doi.org/10.1016/S0167-2738(00)00708-6
Liao YH, Zhou DY, Rao MM, Li WS, Cai ZP, Liang Y, Tan CL (2009) Self-supported poly(methyl methacrylate–acrylonitrile–vinyl acetate)-based gel electrolyte for lithium ion battery. J Power Sources 189(1):139–144. https://doi.org/10.1016/j.jpowsour.2008.10.027
Aravindan V, Vickraman P, Prem Kumar T (2007) ZrO2 nanofiller incorporated PVC/PVdF blend-based composite polymer electrolytes (CPE) complexed with LiBOB. J Membr Sci 305(1-2):146–151. https://doi.org/10.1016/j.memsci.2007.07.044
Li WL, Xing YJ, Xing XY, Li YH, Yang G, Xu LX (2013) PVDF-based composite microporous gel polymer electrolytes containing a novel single ionic conductor SiO2(li+). Electrochim Acta 112:183–190. https://doi.org/10.1016/j.electacta.2013.08.179
Subramania A, Sundaram NTK, Kumar GV (2006) Structural and electrochemical properties of micro-porous polymer blend electrolytes based on PVdF-co-HFP-PAN for Li-ion battery applications. J Power Sources 153(1):177–182. https://doi.org/10.1016/j.jpowsour.2004.12.009
Sundaram NTK, Musthafa OTM, Lokesh KS, Subramania A (2008) Effect of porosity on PVdF-co-HFP–PMMA-based electrolyte. Mater Chem Phys 110(1):11–16. https://doi.org/10.1016/j.matchemphys.2007.12.024
Roghanizad F, Rafizadeh M (2015) Ionic conductivity and interfacial resistance of electrospun poly(acrylonitrile)/poly(methyl methacrylate) fibrous membrane-based polymer electrolytes for lithium ion batteries. Ionics 21(10):2789–2795. https://doi.org/10.1007/s11581-015-1488-x
Shanmukaraj D, Wang GX, Murugan R, Liu HK (2008) Ionic conductivity and electrochemical stability of poly(methylmethacrylate)–poly(ethylene oxide) blend-ceramic fillers composites. J Phys Chem Solids 69(1):243–248. https://doi.org/10.1016/j.jpcs.2007.08.072
Song D, Xu C, Chen Y, He J, Zhao Y, Li P, Lin W, Fu F (2015) Enhanced thermal and electrochemical properties of PVDF-HFP/PMMA polymer electrolyte by TiO2 nanoparticles. Solid State Ionics 282:31–36. https://doi.org/10.1016/j.ssi.2015.09.017
Xu JJ, Ye H (2005) Polymer gel electrolytes based on oligomeric polyether/cross-linked PMMA blends prepared via in situ polymerization. Electrochem Commun 7:829–835
Chen-Yang YW, Chen YT, Chen HC, Lin WT, Tsai CH (2009) Effect of the addition of hydrophobic clay on the electrochemical property of polyacrylonitrile/LiClO4 polymer electrolytes for lithium battery. Polymer 50(13):2856–2862. https://doi.org/10.1016/j.polymer.2009.04.023
Tsutsumi H, Matsuo A, Takase K, Doi S, Hisanaga A, Onimura K, Oishi T (2000) Conductivity enhancement of polyacrylonitrile-based electrolytes by addition of cascade nitrile compounds. J Power Sources 90(1):33–38. https://doi.org/10.1016/S0378-7753(00)00444-4
Wu G, Yang HY, Chen HZ, Yuan F, Yang LG, Wang M, Fu RJ (2007) Novel porous polymer electrolyte based on polyacrylonitrile. Mater Chem Phys 104(2-3):284–287. https://doi.org/10.1016/j.matchemphys.2007.03.013
Carol P, Ramakrishnan P, John B, Cheruvally G (2011) Preparation and characterization of electrospun poly(acrylonitrile) fibrous membrane based gel polymer electrolytes for lithium-ion batteries. J Power Sources 196(23):10156–10162. https://doi.org/10.1016/j.jpowsour.2011.08.037
Wang Q, Song WL, Fan LZ, Song Y (2015) Facile fabrication of polyacrylonitrile/alumina composite membranes based on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes for high-voltage lithium-ion batteries. J Membr Sci 486:21–28. https://doi.org/10.1016/j.memsci.2015.03.022
Phillips SH, Haddad TS, Tomczak SJ (2004) Developments in nanoscience: polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr Opin Solid State Mater Sci 8(1):21–29. https://doi.org/10.1016/j.cossms.2004.03.002
Schwab JJ, Lichtenhan JD, Chaffee KP, Gordon JC, Otonari YA, Carr MJ, Bolf AG (1997) Investigations into structure/property relationships for polyhedral oligomeric silsesquioxane (POSS) based methacrylate polymers. Abs Pap ACS 213:351
Baney RH, Itoh M, Sakakibara A, Suzuki T (1995) Silsesquioxanes. Chem Rev 95(5):1409–1430. https://doi.org/10.1021/cr00037a012
Zhou Z, Cui LM, Zhang Y, Zhang YX, Yin NAW (2008) Preparation and properties of DOSS grafted polypropylene by reactive blending. Eur Polym J 44(10):3057–3066. https://doi.org/10.1016/j.eurpolymj.2008.05.036
Liu YZ, Sun Y, Zeng FL, Zhang QH, Geng L (2014) Characterization and analysis on atomic oxygen resistance of POSS/PVDF composites. Appl Surf Sci 320:908–913. https://doi.org/10.1016/j.apsusc.2014.09.121
Pittman CU Jr, Li GZ, Ni H (2003) Hybrid inorganic/organic crosslinked resins containing polyhedral oligomeric silsesquioxanes. Macromol Symp 196(1):301–325. https://doi.org/10.1002/masy.200390170
Zhang Z, Gu A, Liang G, Ren P, Xie J, Wang X (2007) Thermo-oxygen degradation mechanisms of POSS/epoxy nanocomposites. Polym Degrad Stab 92(11):1986–1993. https://doi.org/10.1016/j.polymdegradstab.2007.08.004
Wang SH, Kuo PL, Hsieh CT, Teng H (2014) Design of poly(acrylonitrile)-based gel electrolytes for high-performance lithium ion batteries. ACS Appl Mater Interfaces 6(21):19360–19370. https://doi.org/10.1021/am505448a
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ionics 177(26-32):2679–2682. https://doi.org/10.1016/j.ssi.2006.04.013
Kim DW, Ko JM, Chun JH (2001) Electrochemical characteristics of Li/LiMn2O4 cells using gel polymer electrolytes. J Power Sources 93(1-2):151–155. https://doi.org/10.1016/S0378-7753(00)00560-7
Kim JR, Choi SW, Jo SM, Lee WS, Kim BC (2004) Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim Acta 50(1):69–75. https://doi.org/10.1016/j.electacta.2004.07.014
Vijayakumar G, Karthick SN, Sathiya Priya AR, Ramalingam S, Subramania A (2008) Effect of nanoscale CeO2 on PVDF-HFP-based nanocomposite porous polymer electrolytes for Li-ion batteries. J Solid State Electrochem 12(9):1135–1141. https://doi.org/10.1007/s10008-007-0460-8
Fasciani C, Panero S, Hassoun J, Scrosati B (2015) Novel configuration of poly(vinylidenedifluoride)-based gel polymer electrolyte for application in lithium-ion batteries. J Power Sources 294:180–186. https://doi.org/10.1016/j.jpowsour.2015.06.068
Zhang Z, Sui G, Bi H, Yang X (2015) Radiation-crosslinked nanofiber membranes with well-designed core–shell structure for high performance of gel polymer electrolytes. J Membr Sci 492:77–87. https://doi.org/10.1016/j.memsci.2015.05.040
Kiran Kumar ABV, Daniel Thangadurai T, Lee YI (2013) Poly [2-(cinnamoyloxy)ethyl methacrylate-co-octamethacryl-POSS] nanocomposites: synthesis and properties. React Funct Polym 73(9):1175–1179. https://doi.org/10.1016/j.reactfunctpolym.2013.05.010
Voronkov MG, Lavrent’yev VI (1982) Polyhedral oligosilsesquioxanes and their homo derivatives. In: Inorganic ring systems, 102 edn. Springer, Berlin Heidelberg, pp 199–236
Dare EO, Liu LK, Peng J (2006) Modified procedure for improved synthesis of some octameric silsesquioxanes via hydrolytic polycondenzation in the presence of amberlite ion-exchange resins. Dalton Trans 30:3668–3671
Tsao CH, Kuo PL (2015) Poly(dimethylsiloxane) hybrid gel polymer electrolytes of a porous structure for lithium ion battery. J Membr Sci 489:36–42. https://doi.org/10.1016/j.memsci.2015.03.087
Wen Y, Lian F, Ren Y, Guan H-Y (2014) Enhanced electrochemical properties of a novel polyvinyl formal membrane supporting gel polymer electrolyte by Al2O3 modification. J Polym Sci B Polym Phys 52(8):572–577. https://doi.org/10.1002/polb.23448
Kim YW, Gong MS, Choi BK (2001) Ionic conduction and electrochemical properties of new poly(acrylonitrile-itaconate)-based gel polymer electrolytes. J Power Sources 97:654–656
Ma X, Huang X, Gao J, Zhang S, Deng Z, Suo J (2014) Compliant gel polymer electrolyte based on poly(methyl acrylate-co-acrylonitrile)/poly(vinyl alcohol) for flexible lithium-ion batteries. Electrochim Acta 115:216–222. https://doi.org/10.1016/j.electacta.2013.10.169
Huang Y, Gong SD, Huang R, Cao HJ, Lin YH, Yang M, Li X (2015) Polyhedral oligomeric silsesquioxane containing gel polymer electrolyte based on a PMMA matrix. RSC Adv 5(57):45908–45918. https://doi.org/10.1039/C5RA06860F
Gong SD, Huang Y, Cao HJ, Lin YH, Li Y, Tang SH, Wang MS, Li X (2016) A green and environment-friendly gel polymer electrolyte with higher performances based on the natural matrix of lignin. J Power Sources 307:624–633. https://doi.org/10.1016/j.jpowsour.2016.01.030
Zhao J, Zhang J, Hu P, Ma J, Wang X, Yue L, Xu G, Qin B, Liu Z, Zhou X, Cui G (2016) A sustainable and rigid-flexible coupling cellulose-supported poly(propylene carbonate) polymer electrolyte towards 5 V high voltage lithium batteries. Electrochim Acta 188:23–30. https://doi.org/10.1016/j.electacta.2015.11.088
Zhou L, Cao Q, Jing B, Wang X, Tang X, Wu N (2014) Study of a novel porous gel polymer electrolyte based on thermoplastic polyurethane/poly(vinylidene fluoride-co-hexafluoropropylene) by electrospinning technique. J Power Sources 263:118–124. https://doi.org/10.1016/j.jpowsour.2014.03.140
Hsu CY, Liu RJ, Hsu CH, Kuo PL (2016) High thermal and electrochemical stability of PVDF-graft-PAN copolymer hybrid PEO membrane for safety reinforced lithium-ion battery. RSC Adv 6(22):18082–18088. https://doi.org/10.1039/C5RA26345J
Acknowledgements
This work was supported by the Key Fund Project of Sichuan Provincial Department of Education (15ZA0050).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 611 kb)
Rights and permissions
About this article
Cite this article
Liu, B., Huang, Y., Cao, H. et al. A novel polyacrylonitrile-based porous structure gel polymer electrolyte composited by incorporating polyhedral oligomeric silsesquioxane by phase inversion method. J Solid State Electrochem 22, 1771–1783 (2018). https://doi.org/10.1007/s10008-017-3877-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3877-8